Spatial Distribution of Chlorophyll a and Its Relationship with the Environment During Summer in Lake Poyang: a Yangtze-Connected Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatiotemporal Evolution of Lakes Under Rapid Urbanization: a Case Study in Wuhan, China
water Article Spatiotemporal Evolution of Lakes under Rapid Urbanization: A Case Study in Wuhan, China Chao Wen 1, Qingming Zhan 1,* , De Zhan 2, Huang Zhao 2 and Chen Yang 3 1 School of Urban Design, Wuhan University, Wuhan 430072, China; [email protected] 2 China Construction Third Bureau Green Industry Investment Co., Ltd., Wuhan 430072, China; [email protected] (D.Z.); [email protected] (H.Z.) 3 College of Urban and Environmental Sciences, Peking University, Beijing 100871, China; [email protected] * Correspondence: [email protected]; Tel.: +86-139-956-686-39 Abstract: The impact of urbanization on lakes in the urban context has aroused continuous attention from the public. However, the long-term evolution of lakes in a certain megacity and the heterogeneity of the spatial relationship between related influencing factors and lake changes are rarely discussed. The evolution of 58 lakes in Wuhan, China from 1990 to 2019 was analyzed from three aspects of lake area, lake landscape, and lakefront ecology, respectively. The Multi-Scale Geographic Weighted Regression model (MGWR) was then used to analyze the impact of related influencing factors on lake area change. The investigation found that the total area of 58 lakes decreased by 15.3%. A worsening trend was found regarding lake landscape with the five landscape indexes of lakes dropping; in contrast, lakefront ecology saw a gradual recovery with variations in the remote sensing ecological index (RSEI) in the lakefront area. The MGWR regression results showed that, on the whole, the increase in Gross Domestic Product (GDP), RSEI in the lakefront area, precipitation, and humidity Citation: Wen, C.; Zhan, Q.; Zhan, contributed to lake restoration. -

Disclosure Guide
WEEKS® 2021 - 2022 DISCLOSURE GUIDE This publication contains information that indicates resorts participating in, and explains the terms, conditions, and the use of, the RCI Weeks Exchange Program operated by RCI, LLC. You are urged to read it carefully. 0490-2021 RCI, TRC 2021-2022 Annual Disclosure Guide Covers.indd 5 5/20/21 10:34 AM DISCLOSURE GUIDE TO THE RCI WEEKS Fiona G. Downing EXCHANGE PROGRAM Senior Vice President 14 Sylvan Way, Parsippany, NJ 07054 This Disclosure Guide to the RCI Weeks Exchange Program (“Disclosure Guide”) explains the RCI Weeks Elizabeth Dreyer Exchange Program offered to Vacation Owners by RCI, Senior Vice President, Chief Accounting Officer, and LLC (“RCI”). Vacation Owners should carefully review Manager this information to ensure full understanding of the 6277 Sea Harbor Drive, Orlando, FL 32821 terms, conditions, operation and use of the RCI Weeks Exchange Program. Note: Unless otherwise stated Julia A. Frey herein, capitalized terms in this Disclosure Guide have the Assistant Secretary same meaning as those in the Terms and Conditions of 6277 Sea Harbor Drive, Orlando, FL 32821 RCI Weeks Subscribing Membership, which are made a part of this document. Brian Gray Vice President RCI is the owner and operator of the RCI Weeks 6277 Sea Harbor Drive, Orlando, FL 32821 Exchange Program. No government agency has approved the merits of this exchange program. Gary Green Senior Vice President RCI is a Delaware limited liability company (registered as 6277 Sea Harbor Drive, Orlando, FL 32821 Resort Condominiums -

Class of 2003 Finals Program
School of Law One Hundred and Seventy-Fourth FINAL EXERCISES The Lawn May 18, 2003 1 Distinction 2 High Distinction 3 Highest Distinction 4 Honors 5 High Honors 6 Highest Honors 7 Distinguished Majors Program School of Law Finals Speaker Mortimer M. Caplin Former Commissioner of the Internal Revenue Service Mortimer Caplin was born in New York in 1916. He came to Charlottesville in 1933, graduating from the College in 1937 and the Law School in 1940. During the Normandy invasion, he served as U.S. Navy beachmaster and was cited as a member of the initial landing force on Omaha Beach. He continued his federal service as Commissioner of the Internal Revenue Service under President Kennedy from 1961 to 1964. When he entered U.Va. at age 17, Mr. Caplin committed himself to all aspects of University life. From 1933-37, he was a star athlete in the University’s leading sport—boxing—achieving an undefeated record for three years in the mid-1930s and winning the NCAA middleweight title in spite of suffering a broken hand. He also served as coach of the boxing team and was president of the University Players drama group. At the School of Law, he was editor-in-chief of the Virginia Law Review and graduated as the top student in his class. In addition to his deep commitment to public service, he is well known for his devotion to teaching and to the educational process and to advancing tax law. Mr. Caplin taught tax law at U.Va. from 1950-61, while serving as president of the Atlantic Coast Conference. -

Spatio-Temporal Distribution Patterns in Environmental Factors, Chlorophyll-A and Microcystins in a Large Shallow Lake, Lake Taihu, China
Int. J. Environ. Res. Public Health 2014, 11, 5155-5169; doi:10.3390/ijerph110505155 OPEN ACCESS International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph Article Spatio-Temporal Distribution Patterns in Environmental Factors, Chlorophyll-a and Microcystins in a Large Shallow Lake, Lake Taihu, China Rui Ye 1, Kun Shan 2,3, Hailong Gao 1, Ruibin Zhang 1, Wen Xiong 1, Yulei Wang 1 and Xin Qian 1,* 1 State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, China; E-Mails: [email protected] (R.Y.); [email protected] (H.G.); [email protected] (R.Z.); [email protected] (W.X.); [email protected] (Y.W.) 2 State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China; E-Mail: [email protected] 3 University of Chinese Academy of Sciences, Beijing 100049, China * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel./Fax: +86-25-8968-0527. Received: 12 March 2014; in revised form: 4 May 2014 / Accepted: 5 May 2014 / Published: 14 May 2014 Abstract: The spatio-temporal distribution of environmental factors, chlorophyll-a (Chl-a), and microcystins (MCs) in a shallow lake, Lake Taihu (China), were investigated from 2009 to 2011 on a monthly basis at nine sampling stations. The annual mean concentration ranges of total nitrogen (TN), total phosphorus (TP), Chl-a, MC-LR and MC-RR were 0.17–10.53 mg/L, 0.027–0.581 mg/L, 0.10–129.75 µg/L, 0.013–2.019 µg/L and + 0.002–0.794 µg/L, respectively. -

Multi-Destination Tourism in Greater Tumen Region
MULTI-DESTINATION TOURISM IN GREATER TUMEN REGION RESEARCH REPORT 2013 MULTI-DESTINATION TOURISM IN GREATER TUMEN REGION RESEARCH REPORT 2013 Greater Tumen Initiative Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH GTI Secretariat Regional Economic Cooperation and Integration in Asia (RCI) Tayuan Diplomatic Compound 1-1-142 Tayuan Diplomatic Office Bldg 1-14-1 No. 1 Xindong Lu, Chaoyang District No. 14 Liangmahe Nanlu, Chaoyang District Beijing, 100600, China Beijing, 100600, China www.tumenprogramme.org www.economicreform.cn Tel: +86-10-6532-5543 Tel: + 86-10-8532-5394 Fax: +86-10-6532-6465 Fax: +86-10-8532-5774 [email protected] [email protected] © 2013 by Greater Tumen Initiative The views expressed in this paper are those of the author and do not necessarily reflect the views and policies of the Greater Tumen Initiative (GTI) or members of its Consultative Commission and Tourism Board or the governments they represent. GTI does not guarantee the accuracy of the data included in this publication and accepts no responsibility for any consequence of their use. By making any designation of or reference to a particular territory or geographic area, or by using the term “country” in this document, GTI does not intend to make any judgments as to the legal or other status of any territory or area. “Multi-Destination Tourism in the Greater Tumen Region” is the report on respective research within the GTI Multi-Destination Tourism Project funded by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. The report was prepared by Mr. James MacGregor, sustainable tourism consultant (ecoplan.net). -
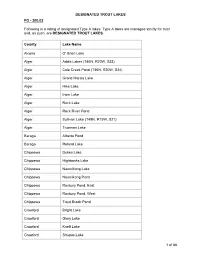
Designated Trout Lakes and Streams
DESIGNATED TROUT LAKES FO - 200.02 Following is a listing of designated Type A lakes. Type A lakes are managed strictly for trout and, as such, are DESIGNATED TROUT LAKES. County Lake Name Alcona O' Brien Lake Alger Addis Lakes (T46N, R20W, S33) Alger Cole Creek Pond (T46N, R20W, S24) Alger Grand Marais Lake Alger Hike Lake Alger Irwin Lake Alger Rock Lake Alger Rock River Pond Alger Sullivan Lake (T49N, R15W, S21) Alger Trueman Lake Baraga Alberta Pond Baraga Roland Lake Chippewa Dukes Lake Chippewa Highbanks Lake Chippewa Naomikong Lake Chippewa Naomikong Pond Chippewa Roxbury Pond, East Chippewa Roxbury Pond, West Chippewa Trout Brook Pond Crawford Bright Lake Crawford Glory Lake Crawford Kneff Lake Crawford Shupac Lake 1 of 86 DESIGNATED TROUT LAKES County Lake Name Delta Bear Lake Delta Carr Lake (T43N, R18W, S36) Delta Carr Ponds (T43N, R18W, S26) Delta Kilpecker Pond (T43N, R18W, S11) Delta Norway Lake Delta Section 1 Pond Delta Square Lake Delta Wintergreen Lake (T43N, R18W, S36) Delta Zigmaul Pond Gogebic Castle Lake Gogebic Cornelia Lake Gogebic Mishike Lake Gogebic Plymouth Lake Houghton Penegor Lake Iron Deadman’s Lk (T41N, R32W, S5 & 8) Iron Fortune Pond (T43N, R33W, S25) Iron Hannah-Webb Lake Iron Killdeer Lake Iron Madelyn Lake Iron Skyline Lake Iron Spree Lake Isabella Blanchard Pond Keweenaw Manganese Lake Keweenaw No Name Pond (T57N, R31W, S8) Luce Bennett Springs Lake Luce Brockies Pond (T46N, R11W, S1) 2 of 86 DESIGNATED TROUT LAKES County Lake Name Luce Buckies Pond (T46N, R11W, S1) Luce Dairy Lake Luce Dillingham -

Chinese Producers and Exporters of Walk-Behind Snow Throwers
Chinese Producers and Exporters of Walk-Behind Snow Throwers 1. Zhejiang Dobest Power Tools Co., T: 86-573-8383-5888 Ltd. F: 86-573-8383-5577 No.9 Huacheng west road,Chengxi New E: [email protected] zone,Yongkang 321300, Zhejiang, China W: http://www.yattool.com/ T: 86-579-89286290 E: [email protected] 7. Zhejiang KC Mechanical & Electrical W: http://www.zjdobest.com/ Co. No.866 East HuaXi Road, 2. Zhejiang Zhouli Industrial Co., Ltd GuShan,YongKang,ZheJiang,China Jinyan Mountain Industry Function Area T: 86-579-87512207 Quanxi,Wuyi,321210 Zhejiang,China E: [email protected] T: 86-579-8798 W: http://www.ykcst.com/ E: [email protected] W: http://chinazhouyi.cn 8. Yongkang Great Power Import and Export Co. Ltd. 3. Century Distribution Systems NO.22 BUILDING,GAOCHUAN 8/F, North Bund Business Center HUAYUAN,JIANGNAN 1050 Dongdaming Road, STREET,YONGKANG JINHAU Hongkou District CITY,ZHEJIANG ZHEJIANG Shanghai 20082, China PROVINCE T: 86-21-5118-3888 F: 86-21-3105-6140 9. Ningbo Vertak Mechanical & W: https://www.cds-net.com/global- Electrical Ltd. offices/ #288 Guangming Road, Zhuangshi Zhenhai District, Ningbo, China 4. Sumec Hardware and Tools Co., Ltd. T: 86-13566024458 No.1, Xinghuo Road, E: [email protected] Nanjing Hi-Tech Zone, W: Nanjing, China https://www.vertak.com/contact/contact. T: 86-25-5863-8000 html F: 86-25-8563-8018 W: www.sumecpower.com 10. Hong Kong Sunrise Trading, Ltd. Rm 3b 5/F Far East 5. Positec (Macao Commercial Office) Consortium Bldg 121 Rm A 8/F, Des Voeux Rd The Macau Sq., Central District, Hong Kong 47 Avenida Do Infan D. -

A Spectral Decomposition Algorithm for Estimating Chlorophyll-A Concentrations in Lake Taihu, China
Remote Sens. 2014, 6, 5090-5106; doi:10.3390/rs6065090 OPEN ACCESS remote sensing ISSN 2072-4292 www.mdpi.com/journal/remotesensing Article A Spectral Decomposition Algorithm for Estimating Chlorophyll-a Concentrations in Lake Taihu, China Yuchao Zhang 1,†, Ronghua Ma 1,*, Hongtao Duan 1,†, Steven Loiselle 2,† and Jinduo Xu 1,† 1 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China; E-Mails: [email protected] (Y.Z.); [email protected] (H.D.); [email protected] (J.X.) 2 Department of Biotechnology, Chemistry and Pharmacy, University of Siena, CSGI, Via Aldo Moro 2, Siena 53100, Italy; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +86-25-8688-2168; Fax: +86-25-5771-4759. Received: 28 March 2014; in revised form: 31 March 2014 / Accepted: 23 May 2014 / Published: 5 June 2014 Abstract: The complex interactions among optically active substances in Case II waters make it difficult to associate the variability in spectral radiance (or reflectance) to any single component. In the present study, we developed a four end-member spectral decomposition model to estimate chlorophyll-a concentrations in a eutrophic shallow lake—Lake Taihu. The new model was constructed by simulated spectral data from Hydrolight and was successfully validated using both of simulated reflectance and in situ reflectance data. Using MEdium Resolution Imaging Spectrometer (MERIS) images, the accuracy of the new model was estimated and compared with other published models. -

Chinese Producers and Exporters of Walk-Behind Lawn Mowers
Chinese Producers and Exporters of Walk-Behind Lawn Mowers 1. Changzhou Globe Co., Ltd. 47 Avenida Do Infan D. Henrique 3-4 65 Xinggang Avenue Macau, Macao Zhhonglou Zone T: 853-853-2871-1715 Changzhou, 213023 China W: www.positecgroup.com T: 86-519-8980-0500 E: [email protected] 7. Changzhou King-Town International W: www.greenworkstools.com.hk No. 50 Juqian Street, Tianning District, 2. Pacific Link International Freight Changzhou, Jiangsu 213000, China (Shanghai) T: 86-519-8816-8225 Room N. 704, E: 86-519-8816-8225 Yaojiang International Mansion No. 308, 8. Zhejiang YAT Electrical Appliance Wu Song Road, Shanghai, China Co. T: 86-21-6031-7066 No. 150 Wenlong Road, F: 86-21-6031-7060 Yuxin Town, E: [email protected] South Lake Zone, Jiaxing 314009, Zhejiang, China 3. Century Distribution Systems T: 86-573-8383-5888 8/F, North Bund Business Center F: 86-573-8383-5577 1050 Dongdaming Road, E: [email protected] Hongkou District W: http://www.yattool.com/ Shanghai 20082, China T: 86-21-5118-3888 9. Scanwell Container Line Ltd. F: 86-21-3105-6140 Room 1002-1003, Block K, Lane 168, W: https://www.cds-net.com/global- The North ChangFeng, Daduhe Road, offices/ Shanghai 200062, China T: 86-25-8473-2035 4. Sumec Hardware and Tools Co., Ltd. W: No.1, Xinghuo Road, http://www.scanwell.com/page?page=G Nanjing Hi-Tech Zone, eneral Ocean Import Nanjing, China T: 86-25-5863-8000 10. Fujian Forestry Materials Co., Ltd. F: 86-25-8563-8018 Qianjiashan Industrial Zone, W: www.sumecpower.com Qingkou Town, Minhou County, Fuzhou, 350119, China 5. -

Chinese Producers and Exporters of Walk-Behind Snow Throwers
Chinese ProducersBarcode:4104620-04 and Exporters A-570-141 of INV Walk-Behind - Investigation Snow - Throwers 1. Zhejiang Dobest Power Tools Co., T: 86-573-8383-5888 Ltd. F: 86-573-8383-5577 No.9 Huacheng west road,Chengxi New E: [email protected] zone,Yongkang 321300, Zhejiang, China W: http://www.yattool.com/ T: 86-579-89286290 E: [email protected] 7. Zhejiang KC Mechanical & Electrical W: http://www.zjdobest.com/ Co. No.866 East HuaXi Road, 2. Zhejiang Zhouli Industrial Co., Ltd GuShan,YongKang,ZheJiang,China Jinyan Mountain Industry Function Area T: 86-579-87512207 Quanxi,Wuyi,321210 Zhejiang,China E: [email protected] T: 86-579-8798 W: http://www.ykcst.com/ E: [email protected] W: http://chinazhouyi.cn 8. Yongkang Great Power Import and Export Co. Ltd. 3. Century Distribution Systems NO.22 BUILDING,GAOCHUAN 8/F, North Bund Business Center HUAYUAN,JIANGNAN 1050 Dongdaming Road, STREET,YONGKANG JINHAU Hongkou District CITY,ZHEJIANG ZHEJIANG Shanghai 20082, China PROVINCE T: 86-21-5118-3888 F: 86-21-3105-6140 9. Ningbo Vertak Mechanical & W: https://www.cds-net.com/global- Electrical Ltd. offices/ #288 Guangming Road, Zhuangshi Zhenhai District, Ningbo, China 4. Sumec Hardware and Tools Co., Ltd. T: 86-13566024458 No.1, Xinghuo Road, E: [email protected] Nanjing Hi-Tech Zone, W: Nanjing, China https://www.vertak.com/contact/contact. T: 86-25-5863-8000 html F: 86-25-8563-8018 W: www.sumecpower.com 10. Hong Kong Sunrise Trading, Ltd. Rm 3b 5/F Far East 5. Positec (Macao Commercial Office) Consortium Bldg 121 Rm A 8/F, Des Voeux Rd The Macau Sq., Central District, Hong Kong 47 Avenida Do Infan D. -

Charted Lakes List
LAKE LIST United States and Canada Bull Shoals, Marion (AR), HD Powell, Coconino (AZ), HD Gull, Mono Baxter (AR), Taney (MO), Garfield (UT), Kane (UT), San H. V. Eastman, Madera Ozark (MO) Juan (UT) Harry L. Englebright, Yuba, Chanute, Sharp Saguaro, Maricopa HD Nevada Chicot, Chicot HD Soldier Annex, Coconino Havasu, Mohave (AZ), La Paz HD UNITED STATES Coronado, Saline St. Clair, Pinal (AZ), San Bernardino (CA) Cortez, Garland Sunrise, Apache Hell Hole Reservoir, Placer Cox Creek, Grant Theodore Roosevelt, Gila HD Henshaw, San Diego HD ALABAMA Crown, Izard Topock Marsh, Mohave Hensley, Madera Dardanelle, Pope HD Upper Mary, Coconino Huntington, Fresno De Gray, Clark HD Icehouse Reservior, El Dorado Bankhead, Tuscaloosa HD Indian Creek Reservoir, Barbour County, Barbour De Queen, Sevier CALIFORNIA Alpine Big Creek, Mobile HD DeSoto, Garland Diamond, Izard Indian Valley Reservoir, Lake Catoma, Cullman Isabella, Kern HD Cedar Creek, Franklin Erling, Lafayette Almaden Reservoir, Santa Jackson Meadows Reservoir, Clay County, Clay Fayetteville, Washington Clara Sierra, Nevada Demopolis, Marengo HD Gillham, Howard Almanor, Plumas HD Jenkinson, El Dorado Gantt, Covington HD Greers Ferry, Cleburne HD Amador, Amador HD Greeson, Pike HD Jennings, San Diego Guntersville, Marshall HD Antelope, Plumas Hamilton, Garland HD Kaweah, Tulare HD H. Neely Henry, Calhoun, St. HD Arrowhead, Crow Wing HD Lake of the Pines, Nevada Clair, Etowah Hinkle, Scott Barrett, San Diego Lewiston, Trinity Holt Reservoir, Tuscaloosa HD Maumelle, Pulaski HD Bear Reservoir, -

Flooding Events Assessment Using Mike Flood Model, Dongting Lake, China
International Journal of Engineering Innovation & Research Volume 5, Issue 1, ISSN: 2277 – 5668 Flooding Events Assessment Using Mike Flood Model, Dongting Lake, China Telesphore Habiyakare a, b ZHOU Nian-qing SHEN Xinping a Department of Hydraulic Engineering Department of Hydraulic Engineering Dongting Lake Water Resources Tongji University, Shanghai 200092, China Tongji University, Shanghai 200092, Administration Bureau of Hunan bCollege of Science and Technology, China Province, Changsha University of Rwanda, Email: [email protected] 410007,China Po Box 1514 Kigali, Rwanda Email: [email protected] Email: [email protected] Abstract – Dongting Lake Basin is composed of a complex representation of the rather complex flow on extended flow regime of rivers network, in recent years a large number flood plains. of floods occurred in the Dongting Lake Basin, Lake water This study has been conducted on the Dongting Lake level crisis, flood prevention and control have become a focus Basin located in and is located in the northeastern part of in the middle Yangtze River. However Numerical modelling Hunan Province, China. Dongting Lake is a large, shallow of flood events often requires a simultaneous description of 2 the flow through relatively narrow channel networks and and covers a water surface area of 2,625 km . It is a flood across extended plains. MIKE FLOOD integrates the one- basin of the Yangtze River. Hence the lake's size depends dimensional models, MIKE 11 for rivers system and the two- on the season. In the July–September period, flood water dimensional model MIKE21 for lake into a single, from the Yangtze flows into the lake, enlarging it greatly, dynamically coupled modelling system.