Abstract Niche Differentiation Among Malagasy Carnivorans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
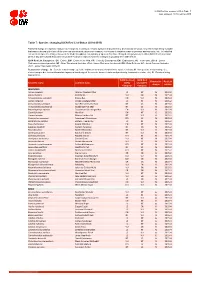
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Galidictis Grandidieri, Grandidier's Vontsira
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T8834A45198057 Galidictis grandidieri, Grandidier’s Vontsira Assessment by: Hawkins, F. View on www.iucnredlist.org Citation: Hawkins, F. 2015. Galidictis grandidieri. The IUCN Red List of Threatened Species 2015: e.T8834A45198057. http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T8834A45198057.en Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: BirdLife International; Botanic Gardens Conservation International; Conservation International; Microsoft; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; Wildscreen; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Chordata Mammalia Carnivora Eupleridae Taxon Name: Galidictis grandidieri Wozencraft, 1986 Common Name(s): • English: Grandidier’s Vontsira, Giant-striped Mongoose, Grandidier's Mongoose Assessment Information Red List Category & Criteria: Endangered B1ab(i,ii,iii,v) ver 3.1 Year Published: 2015 Date Assessed: March 2, 2015 Justification: This species is listed as Endangered under B1ab(i,ii,iii,v). -

The Impact of Forest Logging and Fragmentation on Carnivore Species Composition, Density and Occupancy in Madagascar’S Rainforests
The impact of forest logging and fragmentation on carnivore species composition, density and occupancy in Madagascar’s rainforests B RIAN D. GERBER,SARAH M. KARPANTY and J OHNY R ANDRIANANTENAINA Abstract Forest carnivores are threatened globally by Introduction logging and forest fragmentation yet we know relatively little about how such change affects predator populations. arnivores are one of the most threatened groups of 2009 This is especially true in Madagascar, where carnivores Cterrestrial mammals (Karanth & Chellam, ). have not been extensively studied. To understand better the Declines of predators are often attributed to habitat loss effects of logging and fragmentation on Malagasy carnivores and fragmentation but few quantitative studies have we evaluated species composition, density of fossa examined how carnivore populations and communities 2002 Cryptoprocta ferox and Malagasy civet Fossa fossana, and change with habitat loss or fragmentation (Crooks, ; 2005 carnivore occupancy in central-eastern Madagascar. We Michalski & Peres, ). This is particularly true for ’ photographically-sampled carnivores in two contiguous Madagascar s carnivores, with knowledge lacking about ff (primary and selectively-logged) and two fragmented rain- their ecology and the e ects of anthropogenic disturbances 2010 forests (fragments , 2.5 and . 15 km from intact forest). (Irwin et al., ), especially in the eastern rainforest where Species composition varied, with more native carnivores in only short-term studies have been conducted (Gerber et al., 2010 16 the contiguous than fragmented rainforests. F. fossana was ). With only % of the original primary forests extant absent from fragmented rainforests and at a lower density in Madagascar and those remaining becoming smaller and 2007 in selectively-logged than in primary rainforest (mean more isolated over time (Harper et al., ), habitat loss −2 1.38 ± SE 0.22 and 3.19 ± SE 0.55 individuals km , respect- and fragmentation are serious threats to many endemic 2010 ively). -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Redalyc.TRENDS in RESEARCH on TERRESTRIAL SPECIES of THE
Mastozoología Neotropical ISSN: 0327-9383 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Pérez-Irineo, Gabriela; Santos-Moreno, Antonio TRENDS IN RESEARCH ON TERRESTRIAL SPECIES OF THE ORDER CARNIVORA Mastozoología Neotropical, vol. 20, núm. 1, 2013, pp. 113-121 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Available in: http://www.redalyc.org/articulo.oa?id=45728549008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Mastozoología Neotropical, 20(1):113-121, Mendoza, 2013 Copyright ©SAREM, 2013 Versión impresa ISSN 0327-9383 http://www.sarem.org.ar Versión on-line ISSN 1666-0536 Artículo TRENDS IN RESEARCH ON TERRESTRIAL SPECIES OF THE ORDER CARNIVORA Gabriela Pérez-Irineo and Antonio Santos-Moreno Laboratorio de Ecología Animal, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Oaxaca, Instituto Politécnico Nacional, Hornos 1003, 71230 Santa Cruz Xoxocotlán, Oaxaca, México [Correspondence: Gabriela Pérez Irineo <[email protected]>]. ABSTRACT. Information regarding trends in research on terrestrial species of the order Carnivora can provide an understanding of the degree of knowledge of the order, or lack thereof, as well as help identifying areas on which to focus future research efforts. With the aim of providing information on these trends, this work presents a review of the thematic focuses of studies addressing this order published over the past three de- cades. Relevant works published in 16 scientific journals were analyzed globally and by continent with respect of topics, species, and families. -

Introduction to Camera Trapping
Choosing the right camera traps based on interests, goals, and species Marcella J. Kelly- Professor, Virginia Tech Dept of Fish and Wildlife Conservation WildLabs Community – Tech Tutors July 15, 2021 Remote-Camera Trapping Background Remote cameras/camera traps/game cameras Been around since the late 1890s. But using trip wires and track pads and gave single shots only. 1980s deer hunters => scout hunting grounds 1990s biologists expanded techniques using multiple shot film cameras - film 2000s (mid) brought affordable digital camera technology Remote Camera Applications - Today Scientific Studies Mammals – especially for monitoring of various forest carnivores (e.g. American marten, fisher, wolverine, lynxes, tigers, jaguars, etc.), but also for big game, and large-mammal movement across highways, prey studies, denning behavior (black bears); physical condition of animals (sun bears) Birds – count and monitor ground bird; avian nest predation Herps: e.g. monitoring of timber rattlesnakes. But few herp studies. Remote Wildlife Photography Recreational users (e.g. hunters etc.) Camera Types Cameras now use mostly passive (PIR) infrared sensors PIR –triggers by motion/heat differential when moving object differs in temperature from the environment and moves in front of the sensor Up and coming– remotely download to a base station or satellite uplink Things to consider Do you need protection from wildlife? White flash or infrared? Do you need to lock cameras due to theft? User-friendliness? Do you have a price range? Still photos or video? How long do you need them to last? Protection from the weather? One camera or two per station? How often can you checK them? Battery life Memory card size Kelly et al. -

The Biogeography of Large Islands, Or How Does the Size of the Ecological Theater Affect the Evolutionary Play
The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play Egbert Giles Leigh, Annette Hladik, Claude Marcel Hladik, Alison Jolly To cite this version: Egbert Giles Leigh, Annette Hladik, Claude Marcel Hladik, Alison Jolly. The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play. Revue d’Ecologie, Terre et Vie, Société nationale de protection de la nature, 2007, 62, pp.105-168. hal-00283373 HAL Id: hal-00283373 https://hal.archives-ouvertes.fr/hal-00283373 Submitted on 14 Dec 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THE BIOGEOGRAPHY OF LARGE ISLANDS, OR HOW DOES THE SIZE OF THE ECOLOGICAL THEATER AFFECT THE EVOLUTIONARY PLAY? Egbert Giles LEIGH, Jr.1, Annette HLADIK2, Claude Marcel HLADIK2 & Alison JOLLY3 RÉSUMÉ. — La biogéographie des grandes îles, ou comment la taille de la scène écologique infl uence- t-elle le jeu de l’évolution ? — Nous présentons une approche comparative des particularités de l’évolution dans des milieux insulaires de différentes surfaces, allant de la taille de l’île de La Réunion à celle de l’Amé- rique du Sud au Pliocène. -

The 2008 IUCN Red Listings of the World's Small Carnivores
The 2008 IUCN red listings of the world’s small carnivores Jan SCHIPPER¹*, Michael HOFFMANN¹, J. W. DUCKWORTH² and James CONROY³ Abstract The global conservation status of all the world’s mammals was assessed for the 2008 IUCN Red List. Of the 165 species of small carni- vores recognised during the process, two are Extinct (EX), one is Critically Endangered (CR), ten are Endangered (EN), 22 Vulnerable (VU), ten Near Threatened (NT), 15 Data Deficient (DD) and 105 Least Concern. Thus, 22% of the species for which a category was assigned other than DD were assessed as threatened (i.e. CR, EN or VU), as against 25% for mammals as a whole. Among otters, seven (58%) of the 12 species for which a category was assigned were identified as threatened. This reflects their attachment to rivers and other waterbodies, and heavy trade-driven hunting. The IUCN Red List species accounts are living documents to be updated annually, and further information to refine listings is welcome. Keywords: conservation status, Critically Endangered, Data Deficient, Endangered, Extinct, global threat listing, Least Concern, Near Threatened, Vulnerable Introduction dae (skunks and stink-badgers; 12), Mustelidae (weasels, martens, otters, badgers and allies; 59), Nandiniidae (African Palm-civet The IUCN Red List of Threatened Species is the most authorita- Nandinia binotata; one), Prionodontidae ([Asian] linsangs; two), tive resource currently available on the conservation status of the Procyonidae (raccoons, coatis and allies; 14), and Viverridae (civ- world’s biodiversity. In recent years, the overall number of spe- ets, including oyans [= ‘African linsangs’]; 33). The data reported cies included on the IUCN Red List has grown rapidly, largely as on herein are freely and publicly available via the 2008 IUCN Red a result of ongoing global assessment initiatives that have helped List website (www.iucnredlist.org/mammals). -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -
Download PDF File
1.08 1.19 1.46 Nimravus brachyops Nandinia binotata Neofelis nebulosa 115 Panthera onca 111 114 Panthera atrox 113 Uncia uncia 116 Panthera leo 112 Panthera pardus Panthera tigris Lynx issiodorensis 220 Lynx rufus 221 Lynx pardinus 222 223 Lynx canadensis Lynx lynx 119 Acinonyx jubatus 110 225 226 Puma concolor Puma yagouaroundi 224 Felis nigripes 228 Felis chaus 229 Felis margarita 118 330 227 331Felis catus Felis silvestris 332 Otocolobus manul Prionailurus bengalensis Felis rexroadensis 99 117 334 335 Leopardus pardalis 44 333 Leopardus wiedii 336 Leopardus geoffroyi Leopardus tigrinus 337 Pardofelis marmorata Pardofelis temminckii 440 Pseudaelurus intrepidus Pseudaelurus stouti 88 339 Nimravides pedionomus 442 443 Nimravides galiani 22 338 441 Nimravides thinobates Pseudaelurus marshi Pseudaelurus validus 446 Machairodus alberdiae 77 Machairodus coloradensis 445 Homotherium serum 447 444 448 Smilodon fatalis Smilodon gracilis 66 Pseudaelurus quadridentatus Barbourofelis morrisi 449 Barbourofelis whitfordi 550 551 Barbourofelis fricki Barbourofelis loveorum Stenogale Hemigalus derbyanus 554 555 Arctictis binturong 55 Paradoxurus hermaphroditus Genetta victoriae 553 558 Genetta maculata 559 557 660 Genetta genetta Genetta servalina Poiana richardsonii 556 Civettictis civetta 662 Viverra tangalunga 661 663 552 Viverra zibetha Viverricula indica Crocuta crocuta 666 667 Hyaena brunnea 665 Hyaena hyaena Proteles cristata Fossa fossana 664 669 770 Cryptoprocta ferox Salanoia concolor 668 772 Crossarchus alexandri 33 Suricata suricatta 775 -

Evolutionary History of Carnivora (Mammalia, Laurasiatheria) Inferred
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.05.326090; this version posted October 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. It is not subject to copyright under 17 USC 105 and is also made available for use under a CC0 license. 1 Manuscript for review in PLOS One 2 3 Evolutionary history of Carnivora (Mammalia, Laurasiatheria) inferred 4 from mitochondrial genomes 5 6 Alexandre Hassanin1*, Géraldine Véron1, Anne Ropiquet2, Bettine Jansen van Vuuren3, 7 Alexis Lécu4, Steven M. Goodman5, Jibran Haider1,6,7, Trung Thanh Nguyen1 8 9 1 Institut de Systématique, Évolution, Biodiversité (ISYEB), Sorbonne Université, 10 MNHN, CNRS, EPHE, UA, Paris. 11 12 2 Department of Natural Sciences, Faculty of Science and Technology, Middlesex University, 13 United Kingdom. 14 15 3 Centre for Ecological Genomics and Wildlife Conservation, Department of Zoology, 16 University of Johannesburg, South Africa. 17 18 4 Parc zoologique de Paris, Muséum national d’Histoire naturelle, Paris. 19 20 5 Field Museum of Natural History, Chicago, IL, USA. 21 22 6 Department of Wildlife Management, Pir Mehr Ali Shah, Arid Agriculture University 23 Rawalpindi, Pakistan. 24 25 7 Forest Parks & Wildlife Department Gilgit-Baltistan, Pakistan. 26 27 28 * Corresponding author. E-mail address: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.10.05.326090; this version posted October 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. -

Page 1 SMALL CARNIVORE CONSERVATION INNL The
Svran CnnNrvonr CoNSERVATToN The Newsletterand Journal of the IUCN/SSC Mustelid,Viverrid & ProcyonidSpecialist Group IUCN Number28 April2003 SPEcrEsSunvrvAL CoMMISSIoN Stflpe-necked/vlongoose Herp€stes vitticollis - Photo:M. N.loyokunor, lFS,ARPS, AFIAP The productionand distributionof this issuehas beensponsored by "l\,4arwellPreservation Trust Ltd", Colden Common, UK "RoyalZoological Society of Antwerp",Antwerp, Belgium "CarnivoreConservation & ResearchTrust". Knoxville. TN. USA "ColumbusZoo", Powell, Ohio, USA and "WildlifeConservation Society/Central Park Wildlife Centel', NewYork, NY, USA ColumbusZOO ...1'!hiigtr4rr iihtr r.ontt. t.t dos Svrnn Cnnxrvonr CoNSERVATToN The Newsletterand Joumal of the IUCN/SSC Mustelid.Viverrid & hocyonidSpecialist Group Editor-in-chief: HarryVan Rompaey, Edegem, Belgium Associateeditor: William Duckworth,Bristol, UK Editorial board: AngelaGlatston, Rotterdam, Netherlands MichaelRiffel, Heidelberg,Germany Amd Schreiber,Heidelberg, Germany RolandWinh, Miinchen, Germany Thc !icws cxpressedin tbis publicalioDare thoseoflhe authorsand do not necessarilyreflec! lhose of lhe IUCN. nor the IUCN/SSC Musteljd. viverrid & ProcyonidSpecialist Group. Thc ann ofthis publicatbn is to offc. thc mcmbcrsol rheIUCN/SSC MV&PSG, andthose who are conccmedwith mustclids,vi!cnids, and procyonids,briefpapers, news items. abstacts. and titles of recentliterature. A1l readersare invited to send material toi Small Carnivore Conservation cy'oDr. H. Van Rompaey Jan Verbertlei,15 2650 Edegem- Belgium [email protected] Pnnredon reclcledpaper ISSN 1019-5041 Brown-tailed Mongoose Salanois concolor in the Betampona Reserve, eastern Madagascar:Photographs and an ecological cornparison with Ring-tailed Mongoose Galidia elegans Adam BRITTT and Mcki VIRKAITISI Fi! 1 Bt \tn 1orl..l Moh(..t SaL3i.,a .oicolor Ph.b: V Vnkniis Abstract Photographsot' the lir(le knoNn Bros,n tailed Mongoose Sa I un o i a tu no lor in rhe BetamponaReser!c.