NOAA Technical Memorandum NMFS-NWFSC-109. Technical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pleuronectidae
FAMILY Pleuronectidae Rafinesque, 1815 - righteye flounders [=Heterosomes, Pleronetti, Pleuronectia, Diplochiria, Poissons plats, Leptosomata, Diprosopa, Asymmetrici, Platessoideae, Hippoglossoidinae, Psettichthyini, Isopsettini] Notes: Hétérosomes Duméril, 1805:132 [ref. 1151] (family) ? Pleuronectes [latinized to Heterosomi by Jarocki 1822:133, 284 [ref. 4984]; no stem of the type genus, not available, Article 11.7.1.1] Pleronetti Rafinesque, 1810b:14 [ref. 3595] (ordine) ? Pleuronectes [published not in latinized form before 1900; not available, Article 11.7.2] Pleuronectia Rafinesque, 1815:83 [ref. 3584] (family) Pleuronectes [senior objective synonym of Platessoideae Richardson, 1836; family name sometimes seen as Pleuronectiidae] Diplochiria Rafinesque, 1815:83 [ref. 3584] (subfamily) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Poissons plats Cuvier, 1816:218 [ref. 993] (family) Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Leptosomata Goldfuss, 1820:VIII, 72 [ref. 1829] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Diprosopa Latreille, 1825:126 [ref. 31889] (family) Platessa [no stem of the type genus, not available, Article 11.7.1.1] Asymmetrici Minding, 1832:VI, 89 [ref. 3022] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Platessoideae Richardson, 1836:255 [ref. 3731] (family) Platessa [junior objective synonym of Pleuronectia Rafinesque, 1815, invalid, Article 61.3.2 Hippoglossoidinae Cooper & Chapleau, 1998:696, 706 [ref. 26711] (subfamily) Hippoglossoides Psettichthyini Cooper & Chapleau, 1998:708 [ref. 26711] (tribe) Psettichthys Isopsettini Cooper & Chapleau, 1998:709 [ref. 26711] (tribe) Isopsetta SUBFAMILY Atheresthinae Vinnikov et al., 2018 - righteye flounders GENUS Atheresthes Jordan & Gilbert, 1880 - righteye flounders [=Atheresthes Jordan [D. -

Pacific, Northeast
536 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Northeast C-67 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, nord-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, nordeste English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2012 2013 2014 2015 2016 2017 2018 Nombre inglés Nombre científico Grupo de especies t t t t t t t White sturgeon Acipenser transmontanus 21 76 64 27 21 23 36 26 Pink(=Humpback) salmon Oncorhynchus gorbuscha 23 107 935 327 085 146 932 278 246 62 659 228 336 61 591 Chum(=Keta=Dog) salmon Oncorhynchus keta 23 74 254 78 418 44 746 68 310 61 935 86 782 62 944 Sockeye(=Red) salmon Oncorhynchus nerka 23 100 038 81 848 141 311 135 474 132 554 132 610 120 341 Chinook(=Spring=King) salmon Oncorhynchus tshawytscha 23 7 543 9 067 11 894 9 582 6 991 5 251 3 203 Coho(=Silver) salmon Oncorhynchus kisutch 23 11 470 19 716 23 167 12 241 14 699 17 170 13 100 Pacific salmons nei Oncorhynchus spp 23 ... ... ... ... ... ... 17 586 Eulachon Thaleichthys pacificus 23 - - 9 6 2 1 ... Smelts nei Osmerus spp, Hypomesus spp 23 335 213 280 261 162 162 159 Salmonoids nei Salmonoidei 23 1 1 0 1 1 - 1 American shad Alosa sapidissima 24 152 23 52 51 94 95 179 Pacific halibut Hippoglossus stenolepis 31 19 048 17 312 14 098 14 718 15 033 15 707 13 207 English sole Pleuronectes vetulus 31 202 367 295 319 393 204 304 Greenland halibut Reinhardtius hippoglossoides 31 4 621 1 394 1 397 2 084 2 156 2 733 1 760 -

Methodology of the Pacific Marine Ecological Classification System and Its Application to the Northern and Southern Shelf Bioregions
Canadian Science Advisory Secretariat (CSAS) Research Document 2016/035 Pacific Region Methodology of the Pacific Marine Ecological Classification System and its Application to the Northern and Southern Shelf Bioregions Emily Rubidge1, Katie S. P. Gale1, Janelle M. R. Curtis2, Erin McClelland3, Laura Feyrer4, Karin Bodtker5, Carrie Robb5 1Institute of Ocean Sciences Fisheries & Oceans Canada P.O. Box 6000 Sidney, BC V8L 4B2 2Pacific Biological Station Fisheries & Oceans Canada 3190 Hammond Bay Rd Nanaimo, BC V9T 1K6 3EKM Scientific Consulting 4BC Ministry of Environment P.O. Box 9335 STN PROV GOVT Victoria, BC V8W 9M1 5Living Oceans Society 204-343 Railway St. Vancouver, BC V6A 1A4 May 2016 Foreword This series documents the scientific basis for the evaluation of aquatic resources and ecosystems in Canada. As such, it addresses the issues of the day in the time frames required and the documents it contains are not intended as definitive statements on the subjects addressed but rather as progress reports on ongoing investigations. Research documents are produced in the official language in which they are provided to the Secretariat. Published by: Fisheries and Oceans Canada Canadian Science Advisory Secretariat 200 Kent Street Ottawa ON K1A 0E6 http://www.dfo-mpo.gc.ca/csas-sccs/ [email protected] © Her Majesty the Queen in Right of Canada, 2016 ISSN 1919-5044 Correct citation for this publication: Rubidge, E., Gale, K.S.P., Curtis, J.M.R., McClelland, E., Feyrer, L., Bodtker, K., and Robb, C. 2016. Methodology of the Pacific Marine Ecological Classification System and its Application to the Northern and Southern Shelf Bioregions. -

Flounders, Halibuts, Soles Capture Production by Species, Fishing Areas
95 Flounders, halibuts, soles Capture production by species, fishing areas and countries or areas B-31 Flets, flétans, soles Captures par espèces, zones de pêche et pays ou zones Platijas, halibuts, lenguados Capturas por especies, áreas de pesca y países o áreas Species, Fishing area Espèce, Zone de pêche 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Especie, Área de pesca t t t t t t t t t t Leopard flounder Rombou léopard Lenguado leopardo Bothus pantherinus 1,83(01)018,05 OUN 51 Bahrain ... ... ... ... 3 5 0 0 2 2 Saudi Arabia 84 65 84 96 67 86 124 91 91 93 51 Fishing area total 84 65 84 96 70 91 124 91 93 95 Species total 84 65 84 96 70 91 124 91 93 95 Lefteye flounders nei Arnoglosses, rombous nca Rodaballos, rombos, etc. nep Bothidae 1,83(01)XXX,XX LEF 27 Germany 370 290 292 280 385 262 321 24 1 - Portugal 117 107 106 162 224 202 189 117 106 136 Spain ... ... ... ... 49 92 68 63 106 134 27 Fishing area total 487 397 398 442 658 556 578 204 213 270 31 Korea Rep 2 - - - - - - - - - 31 Fishing area total 2 - - - - - - - - - 34 Cameroon 0 - - - - - - - - - Korea Rep 15 - - - - 5 - - - - Portugal 2 12 11 17 7 - - - 15 15 34 Fishing area total 17 12 11 17 7 5 - - 15 15 41 Estonia - - 1 - - - - - - - Korea Rep - 20 - - - - - - - - 41 Fishing area total - 20 1 - - - - - - - 48 UK 0 - - - - - - - - - 48 Fishing area total 0 - - - - - - - - - 51 Eritrea 119 125 69 49 77 20 85 1 0 15 Korea Rep 10 - 19 24 1 - - - 1 - 51 Fishing area total 129 125 88 73 78 20 85 1 1 15 71 Korea Rep 8 10 1 - - - - - - - 71 Fishing area total 8 10 1 - - - -

Flounders, Halibuts, Soles Capture Production by Species, Fishing Areas
101 Flounders, halibuts, soles Capture production by species, fishing areas and countries or areas B-31 Flets, flétans, soles Captures par espèces, zones de pêche et pays ou zones Platijas, halibuts, lenguados Capturas por especies, áreas de pesca y países o áreas Species, Fishing area Espèce, Zone de pêche 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 Especie, Área de pesca t t t t t t t t t t Mediterranean scaldfish Arnoglosse de Méditerranée Serrandell Arnoglossus laterna 1,83(01)001,01 MSF 34 Italy - - - - - - - 57 223 123 34 Fishing area total - - - - - - - 57 223 123 37 Italy ... ... ... ... ... ... 447 479 169 403 37 Fishing area total ... ... ... ... ... ... 447 479 169 403 Species total ... ... ... ... ... ... 447 536 392 526 Leopard flounder Rombou léopard Lenguado leopardo Bothus pantherinus 1,83(01)018,05 OUN 51 Bahrain 2 - - 1 1 4 4 F 4 F 4 F 4 F Saudi Arabia 77 80 77 75 74 83 71 79 80 F 74 51 Fishing area total 79 80 77 76 75 87 75 F 83 F 84 F 78 F Species total 79 80 77 76 75 87 75 F 83 F 84 F 78 F Lefteye flounders nei Arnoglosses, rombous nca Rodaballos, rombos, etc. nep Bothidae 1,83(01)XXX,XX LEF 21 USA 1 087 774 566 747 992 759 545 406 633 409 21 Fishing area total 1 087 774 566 747 992 759 545 406 633 409 27 Germany - - - - - - - - 0 - Portugal 136 103 143 125 105 102 87 76 84 105 Spain 134 116 96 56 29 8 12 12 6 5 27 Fishing area total 270 219 239 181 134 110 99 88 90 110 31 USA 59 38 71 45 41 128 117 133 99 102 31 Fishing area total 59 38 71 45 41 128 117 133 99 102 34 Greece - - - - - - - 71 45 - Portugal 15 46 .. -

Risk Analysis of a Flatfish Stock Complex
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. RISK ANALYSIS OF A FLATFISH STOCK COMPLEX K. McLeod 2010 RISK ANALYSIS OF A FLATFISH STOCK COMPLEX A thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Mathematics at Massey University Kristin McLeod 2010 ii ABSTRACT The New Zealand Ministry of Fisheries relies on fishery assessments to determine suitable catch quotas for exploited fisheries. Currently, 628 fish stocks are managed in New Zealand using the Quote Management System, which includes the 8 com- mercial flatfish species caught within the Exclusive Economic Zone. These eight species of flatfish, which includes four species of flounder, two species of sole, brill and turbot, are currently managed using a combined catch quota. Since these eight species are managed using a common catch quota, there is concern that some of the individual species may be under or over-fished. This thesis describes work involving the flatfish species caught in the FLA3 man- agement area, around the south island of New Zealand. The FLA3 management area contains three key species: New Zealand sole, lemon sole, and sand flounder. Due to the nature and limitations of the data available, simple biomass dynamic models were applied to these species. The maximum likelihood and Bayesian goodness of fit techniques were used to estimate the model parameters. Three models were used: the Fox model, the Schaefer model and the Pella-Tomlinson model with m =3. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

61661147.Pdf
Resource Inventory of Marine and Estuarine Fishes of the West Coast and Alaska: A Checklist of North Pacific and Arctic Ocean Species from Baja California to the Alaska–Yukon Border OCS Study MMS 2005-030 and USGS/NBII 2005-001 Project Cooperation This research addressed an information need identified Milton S. Love by the USGS Western Fisheries Research Center and the Marine Science Institute University of California, Santa Barbara to the Department University of California of the Interior’s Minerals Management Service, Pacific Santa Barbara, CA 93106 OCS Region, Camarillo, California. The resource inventory [email protected] information was further supported by the USGS’s National www.id.ucsb.edu/lovelab Biological Information Infrastructure as part of its ongoing aquatic GAP project in Puget Sound, Washington. Catherine W. Mecklenburg T. Anthony Mecklenburg Report Availability Pt. Stephens Research Available for viewing and in PDF at: P. O. Box 210307 http://wfrc.usgs.gov Auke Bay, AK 99821 http://far.nbii.gov [email protected] http://www.id.ucsb.edu/lovelab Lyman K. Thorsteinson Printed copies available from: Western Fisheries Research Center Milton Love U. S. Geological Survey Marine Science Institute 6505 NE 65th St. University of California, Santa Barbara Seattle, WA 98115 Santa Barbara, CA 93106 [email protected] (805) 893-2935 June 2005 Lyman Thorsteinson Western Fisheries Research Center Much of the research was performed under a coopera- U. S. Geological Survey tive agreement between the USGS’s Western Fisheries -

Fish, Crustaceans, Molluscs, Etc Capture Production by Species
509 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Northeast C-67 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, nord-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, nordeste English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2005 2006 2007 2008 2009 2010 2011 Nombre inglés Nombre científico Grupo de especies t t t t t t t White sturgeon Acipenser transmontanus 21 188 158 143 164 154 120 110 Green sturgeon Acipenser medirostris 21 3 3 0 0 0 0 0 Pink(=Humpback) salmon Oncorhynchus gorbuscha 23 237 008 102 021 218 820 118 527 146 700 170 264 185 423 Chum(=Keta=Dog) salmon Oncorhynchus keta 23 46 553 78 098 54 408 57 924 54 220 53 689 53 003 Sockeye(=Red) salmon Oncorhynchus nerka 23 120 787 118 293 127 553 103 881 116 847 134 749 116 163 Chinook(=Spring=King) salmon Oncorhynchus tshawytscha 23 10 310 8 558 6 951 5 312 5 366 6 978 7 494 Coho(=Silver) salmon Oncorhynchus kisutch 23 16 806 15 634 12 457 16 756 15 699 15 675 12 399 Rainbow trout Oncorhynchus mykiss 23 - 1 1 2 - - - Pacific salmons nei Oncorhynchus spp 23 0 - 0 - 0 - - Eulachon Thaleichthys pacificus 23 0 7 4 8 8 2 0 Smelts nei Osmerus spp, Hypomesus spp 23 153 206 182 218 164 144 199 Salmonoids nei Salmonoidei 23 2 - - - - - 3 American shad Alosa sapidissima 24 234 82 82 19 2 14 - Atlantic halibut Hippoglossus hippoglossus 31 - - - - - 1 - Pacific halibut Hippoglossus stenolepis 31 41 185 39 658 37 748 35 087 31 113 29 730 23 542 English -
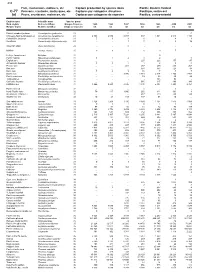
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
434 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Eastern Central C-77 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, centre-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, centro-oriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 1995 1996 1997 1998 1999 2000 2001 Nombre inglés Nombre científico Grupo de especies mt mt mt mt mt mt mt Pink(=Humpback)salmon Oncorhynchus gorbuscha 23 - - 0 - - - 0 Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 2 993 2 078 2 737 937 1 991 2 613 1 193 Coho(=Silver)salmon Oncorhynchus kisutch 23 5 6 3 0 - 4 1 Smelts nei Osmerus spp, Hypomesus spp 23 - - 7 7 5 6 12 American shad Alosa sapidissima 24 - - 0 - - - - Milkfish Chanos chanos 25 0 0 1 0 0 7 ... Lefteye flounders nei Bothidae 31 - - - - 9 - - Pacific halibut Hippoglossus stenolepis 31 3 4 2 3 2 - 0 English sole Pleuronectes vetulus 31 ... ... ... 227 226 177 197 Arrow-tooth flounder Atheresthes stomias 31 1 1 1 1 2 0 0 Petrale sole Eopsetta jordani 31 ... ... 483 267 259 238 268 Rex sole Glyptocephalus zachirus 31 ... ... ... 168 153 128 98 Rock sole Lepidopsetta bilineata 31 7 4 9 10 7 7 7 Dover sole Microstomus pacificus 31 ... ... 3 846 1 818 2 174 1 866 1 403 Pacific sand sole Psettichthys melanostictus 31 ... ... ... 19 23 35 44 Tonguefishes Cynoglossidae 31 - - - - 105 95 - California flounder Paralichthys californicus 31 ... ... ... 510 617 390 389 Flatfishes nei Pleuronectiformes 31 8 466 9 220 4 596 1 830 2 893 3 293 2 350 Pacific tomcod Microgadus proximus 32 .. -

Change in Oregon's Nearshore Groundfish Trawl Fishery
AN ABSTRACT OF THE THESIS OF Rebecca A. Howard for the degree of Master of Science in Marine Resource Management presented on September 9, 2020. Title: Change in Oregon’s Nearshore Groundfish Trawl Fishery: Perspectives from Science and Industry Data. Abstract approved: _____________________________________________________________ Lorenzo Ciannelli The commercial groundfish fishing industry and groundfish research have a long concurrent history of activity on the Oregon continental margin. Within the non-whiting groundfish fishery, the target species are primarily flatfishes, sablefish, lingcod, and rockfishes, though landings of each have fluctuated over time. Recent work shows that over the past two decades, fishing effort has shifted offshore likely due to implementation of gear regulations, area closures, and lower catch limits. Although federal fishery-independent surveys have been conducted across most of the groundfish fishery’s depth range, data is limited by years and seasons surveyed as well as absence of data in the shallowest waters (< 55 m). Fishery-dependent data covers those shallow waters and a broader temporal range, but at a coarse scale. Limitations in data coverage combined with a historical focus on deep-water groundfishes has led to a gap in understanding of dynamics within the nearshore fishery, particularly regarding the influence of environmental factors on abundance and distribution. Through this thesis, I analyzed changes in spatiotemporal dynamics of the Oregon nearshore non-whiting groundfish trawl fishery and assessed gaps in each data source over the past four decades. Statistical modeling was used to elucidate distribution shifts in species as well as temporal changes in community composition. These analyses revealed how individual species’ distributions have geographically shifted over time, what environmental variables affect their spatial distribution, and how depth and habitat type strongly influence nearshore community composition. -

Other Fishes in the Coos Estuary
Other Fishes in the Coos Estuary Summary: • Over 70 species of non-salmonid fish use the Coos system at some point in their lives, including one endemic species (Millicoma dace), yet surprisingly little is known about the ecology, Speckled sanddab population or distribution of Photo: U of OR these fishes. • The Coos system appears to Pacific Staghorn sculpin Photo: the outershores.com provide a variety of habitat types that support high fish species Pacific sand lance richness. Photo: NOAA What’s Happening At least 70 non-salmonid fish species (“other fishes”) inhabit the Coos estuary and associat- ed rivers at some point in their life cycle (see Figure 1. Study locations where Other Fish data were collected. Table 1 for a list of those documented in the last 5 years). “Other” fishes use the estuary zon and lingcod inhabiting the waters around and its tributaries variously: some marine rock jetties); some live most of their adult fishes may live in the lower, more saline life in the ocean, but come into the estuary reaches of the estuary as adults (e.g., Caba- to spawn (e.g. Pacific herring or topsmelt); some spawn in the ocean as adults, but their larvae or juveniles enter the estuary to take advantage of productive nursery habitat (e.g. rockfish living in eelgrass beds). Other fishes live in the brackish waters (e.g. shiner perch) or fresh waters (e.g. stickleback, longnose dace) of the Coos estuary through all stages of their life cycles. Unfortunately, distribution and abundance of Other Fish species in the Coos estuary are poorly understood since these species have not, for the most part, been the subject of targeted studies.