Bark Anatomy and Cell Size Variation in Quercus Faginea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Histology and Anatomy Q
PLANT HISTOLOGY AND ANATOMY Q. Transcellular strands are seen in a) Xylem vessels b) ThidTracheids c) Parenchyma cells d) Sieve tubes Q. Epiphytes absorb water by a spongy tissue called a) Mesophy ll b) Velamen c) Conjuctive tissue d) Phloem Q. The presence of vessel s and companion cells are characters of a) Gymnosperms b) Angiosperms c) Bryophytes d) Pteridophytes Q. AhilAmphivasal vascul lbdlar bundle is found in a) Cycas and Dryopteris b) Dracaena and Yucca c) Helianthus and Cucurbita d) maize and Wheat Q. Bamboo and grasses elonggyate by the activity of a) Apical meristem b) Intercalary meristem c) SdSecondary meritistem d) Lateral ameristem Q. Fibres associated with phloem are called a) Intraxylary fibres b) Pericycle fibres c) Bast fibres d) Cortical fibres Q. Callose is found in a) Sieve Plates b) Cross walls of tracheids c) Phloem parenchyma d) Comapanion cell Q. Which are common in xylem and phloem tissues? a) PhParenchyma and CllCollench yma b) Collenchyma and Sclerenchyma c) Parenchyma and Sclerenchyma d) Aerenchyma and Sclerenchyma Q. Quiescent centre is found in a) Root tip b) Shoot tip c) Floral tip d) Leaf tip Q. The plastids in meristematic tissue are in a a) Fullyyp developed state b) Half developed state c) Proplastid state d) Plasmolysed state Q. In hydrophytes, aerenchyma helps in a) Attachment b) Photosynthesis c) Buoyancy d) Mechanical support Q. Cistoliths are composedfd of a) Calcium oxalate b) Calcium carbonate c) GGucosdeslucosides d) MgCO 3 Q. CllCollench yma differs f rom sclerenchyma a) Retaining protoplasm at maturity b) Having thick walls c) HHiaving w idlide lumen d) Being meristematic Q. -

Microbiology and Plant Pathology
ALAGAPPA UNIVERSITY (Accredited with ‘A+’ Grade by NAAC (with CGPA: 3.64) in the Third Cycle and Graded As category - I University by MHRD-UGC) (A State University Established by the Government of TamilNadu) KARAIKUDI – 630 003 DIRECTORATE OF DISTANCE EDUCATION M. Sc. BOTONY Second Year – Third Semester 34631- MICROBIOLOGY AND PLANT PATHOLOGY Copy Right Reserved For Private Use only Authors: Dr. A. Arun, Associate Professor & Head (i/c), Department of Microbiology, Alagappa University, Karaikudi- 630 003. (Units 1-7) Dr. M. Jothibasu Assistant Professor Department of Botany Alagappa University Karaikudi-03 (Units 8-14) “The Copyright shall be vested with Alagappa University” All rights reserved. No part of this publication which is material protected by this copyright notice may be reproduced or transmitted or utilized or stored in any form or by any means now known or hereinafter invented, electronic, digital or mechanical, including photocopying, scanning, recording or by any information storage or retrieval system, without prior written permission from the Alagappa University, Karaikudi, Tamil Nadu. SYLLABI – BOOK MAPPING TABLE 34631- MICROBIOLOGY AND PLANT PATHOLOGY SYLLABI MAPPING IN BOOK BLOCK-1: SCOPE OF MICROBIOLOGY UNIT I Introduction to Microbiology- Scope of Microbiology- 1-8 Evolution into Science- Characterization of microorganisms. UNIT II Classification of Microorganisms- Archea and Bacteria- 9-15 Characteristics of Bacteria- Morphology -Ultra Structure. UNIT III Nutrition – Growth – Reproduction- Bacterial Cultural and 16-27 Cultural characteristics- Economic importance of bacteria BLOCK 2: VIRUS AND MYCOPLASMAS UNIT IV Virology – General features- Classification of virus- 28-34 Characteristics – Ultra structure. UNIT V Virus- Isolation – Purification –Chemical nature- Replication- 35-46 Transmission- Virions. -

Tissues and Other Levels of Organization MODULE - 1 Diversity and Evolution of Life
Tissues and Other Levels of Organization MODULE - 1 Diversity and Evolution of Life 5 Notes TISSUES AND OTHER LEVELS OF ORGANIZATION You have just learnt that cell is the fundamental structural and functional unit of organisms and that bodies of organisms are made up of cells of various shapes and sizes. Groups of similar cells aggregate to collectively perform a particular function. Such groups of cells are termed “tissues”. This lesson deals with the various kinds of tissues of plants and animals. OBJECTIVES After completing this lesson, you will be able to : z define tissues; z classify plant tissues; z name the various kinds of plant tissues; z enunciate the tunica corpus theory and histogen theory; z classify animal tissues; z describe the structure and function of various kinds of epithelial tissues; z describe the structure and function of various kinds of connective tissues; z describe the structure and function of muscular tissue; z describe the structure and function of nervous tissue. 5.1 WHAT IS A TISSUE Organs such as stem, and roots in plants, and stomach, heart and lungs in animals are made up of different kinds of tissues. A tissue is a group of cells with a common origin, structure and function. Their common origin means they are derived from the same layer (details in lesson No. 20) of cells in the embryo. Being of a common origin, there are similar in structure and hence perform the same function. Several types of tissues organise to form an organ. Example : Blood, bone, and cartilage are some examples of animal tissues whereas parenchyma, collenchyma, xylem and phloem are different tissues present in the plants. -

Tree Identification: from Bark and Leaves Or Needles
Tree Identification: from bark and leaves or needles A walk in the woods can be a lot of fun, especially if you bring your kids. How do you get them to come along with you? Tell them this. “Look at the bark on the trees. Can you can find any that look like burnt potato chips, warts, cat scratches, camouflage pants or rippling muscles?” Believe it or not, these are descriptions of different kinds of tree bark. Tree ID from bark:______________________________________________________ Black cherry: The bark looks like burnt potato chips. Hackberry: The bark is bumpy and warty. Ironwood: The bark has long thin strips. With a little imagination, an ironwood can look like it is used as a scratching post by cats. They can also be easily spotted in winter because their light brown dead leaves hang on well past the first snow. Sycamore: A tree with bark that looks like camouflaged pants. The largest tree in Illinois is a sycamore, a majestic 115-foot tree near Springfield. Musclewood: a tree with smooth gray bark covering a trunk with ridges that look like they are rippling muscles. Shagbark hickory: Long strips of shaggy bark peeling at both ends. Cottonwood: Has heavy ridges that make it look like Paul Bunyan’s corduroy pants. Bur oak: Thick and gnarly bark has deep craggy furrows. The corky bark allows it to easily withstand hot forest fires. Tree ID from leaves or needles:________________________________________ Willow Oak: Has elongated leaves similar to those of a willow tree. Red pine: Red has 3 letters; a red pine has groupings of 2-3 prickly needles. -
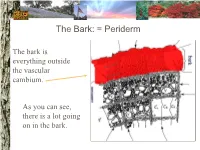
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division. -

Getting Chemicals Into Trees Without Spraying
Urban Forestry NR/FF/020 (pr) Getting Chemicals Into Trees Without Spraying Michael Kuhns, Forestry Extension Specialist This fact sheet provides an overview of injection, or phellogen that makes cork to thicken the outer bark, implantation, and other ways to get chemicals, mainly phloem that conducts food through the tree from where pesticides, into trees. Many techniques and systems it is stored or made to where it is being used (all of these exist and some are very good, some are good in some tissues together make up the bark), vascular cambium situations, and some are ineffective or bad for trees. that divides rapidly to make new phloem and xylem cells, This fact sheet addresses all of these. and xylem or wood. Xylem includes an outer layer called the sapwood that conducts mostly water and minerals from the roots to the canopy, and an inner layer called the Chemicals are applied to trees for many reasons. heartwood that is aged sapwood that has died and has lost Insecticides repel or kill damaging insects, fungicides its ability to conduct water but still adds strength. treat or prevent fungal diseases, nutrients and plant growth regulators affect growth, and herbicides kill trees or prevent sprouting after tree removal. Spraying is the most typical way to apply these chemicals. It is fast, uses readily available equipment, and is understood. The Phellem (Cork Phellogen down side of spraying is that much of the chemical being or Outer Bark) Phloem applied is wasted, either to drift, run off, or because it can not be applied precisely to where it is needed in the tree. -

Tree Anatomy Stems and Branches
Tree Anatomy Series WSFNR14-13 Nov. 2014 COMPONENTSCOMPONENTS OFOF PERIDERMPERIDERM by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Around tree roots, stems and branches is a complex tissue. This exterior tissue is the environmental face of a tree open to all sorts of site vulgarities. This most exterior of tissue provides trees with a measure of protection from a dry, oxidative, heat and cold extreme, sunlight drenched, injury ridden site. The exterior of a tree is both an ecological super highway and battle ground – comfort and terror. This exterior is unique in its attributes, development, and regeneration. Generically, this tissue surrounding a tree stem, branch and root is loosely called bark. The tissues of a tree, outside or more exterior to the xylem-containing core, are varied and complexly interwoven in a relatively small space. People tend to see and appreciate the volume and physical structure of tree wood and dismiss the remainder of stem, branch and root. In reality, tree life is focused within these more exterior thin tissue sets. Outside of the cambium are tissues which include transport cells, structural support cells, generation cells, and cells positioned to help, protect, and sustain other cells. All of this life is smeared over the circumference of a predominately dead physical structure. Outer Skin Periderm (jargon and antiquated term = bark) is the most external of tree tissues providing protection, water conservation, insulation, and environmental sensing. Periderm is a protective tissue generated over and beyond live conducting and non-conducting cells of the food transport system (phloem). -

Why Do Animals Eat the Bark and Wood of Trees and Shrubs? William R
FNR-203 Purdue University & Natural Re ry sou Forestry and Natural Resources st rc re e o s F Why Do Animals Eat the Bark and Wood of Trees and Shrubs? William R. Chaney, Professor of Tree Physiology Department of Forestry and Natural Resources Purdue University, West Lafayette, IN 47907 PURDUE UNIVERSITY Animals gnawing the bark and wood of trees anatomy, movement and storage of food in trees, and shrubs is not a malicious act or evidence of a and the variations in digestive systems among neurotic condition. Instead, it is the normal means animals. by which some animals acquire a nutritious food source. The ability to consume this seemingly Constituents of Plant Cell Walls unpalatable food supply and derive nourishment Unlike animals, plants have cells with rigid from it requires specialized feeding habits and cell walls composed of cellulose, hemicellulose, digestive systems. and lignins. Pectins help hold the individual cells together in tissues. All of these substances are potential sources of food for animals. Cellulose The most consummate wood feeders are the A principal component of cell walls is billions of termites throughout the world that cellulose, which consists of 5,000 to 10,000 literally devour thousands of tons of woody glucose molecules joined by a so-called beta debris every year in forests as well as lumber linkage to form straight chain polymers of pure in buildings. Many of our most serious and sugar. The long chains of cellulose are combined damaging insect pests are the bark beetles and first into microfibrils and then further into wood borers that feed on the parts of trees for macrofibrils to create a mesh-like matrix of cell which they are named. -

Xylem Resin in the Resistance of the Pinaceae to Bark Beetles
XYLEM RESIN IN THE RESISTANCE OF THE PINACEAE TO BARK BEETLES Richard H. Smith Xylem resin-a supersaturation of resin acids in terpenes- appears to play a paradoxical role in the relation of the Pinaceae to tree-killing bark beetles. It has been suggested as the agent responsible for the susceptibility of this coniferous family to beetle attacks. And, at the same time, it has been linked with the ability of the Pinaceae to resist bark beetles. The hosts of tree- killing bark beetles are nearly all Pinaceae-Pinus (pine), Abies (fir), Pseudotsuga (Douglas-fir), Picea (spruce), Tsuga (hemlock), and Larix (larch). Significantly, among conifers, xylem resin is most common and abundant in these Pinaceae. Bark beetles are found on other families of conifers, but they are usually con- sidered of minor importance. Much experimental work has been done since the early reports of an apparent association of resin with resistance. Nearly all studies have dealt with attacking adult beetles; virtually no re- search has been directed at effects of resin on immature forms. Most work has been with Pinus and Dep'roctonus, with lesser attention to spruce, fir, Douglas-fir, and the other genera of bark beetles. PACIFIC This report summarizes the early findings, updates them with results of more recent reports and, in some cases, reinterprets SOUTHWEST these previous reports. This review deals only with bark beetles that attack living trees and with xylem resin, although in a few Forest and Ranee instances, it concerns resin-related chemicals as well. This review follows the approach of Painter (1951), who pro- Experiment station posed that plant resistance to insects depends on one or more FOREST SERVICE U. -

The Anatomy As a Tool for the Identification of the Bark of Pterocarpus Angolensis and Terminalia Sericea1 a Anatomia Como Ferra
The anatomy as a tool for the identification of the bark of Pterocarpus angolensis and Terminalia sericea1 Teresa Quilhó 2* Fernanda Bessa2 Ana Isabel Ribeiro-Barros3 Natasha Ribeiro4 1Selected from the IV Brazilian Congress of Wood Science and Technology 2 Universidade de Lisboa, Instituto Superior de Agronomia, Centro de Estudos Florestais, Tapada da Ajuda, 1349-017 Lisboa, Portugal 3 Universidade de Lisboa, Instituto Superior de Agronomia, Centro de Investigação em Agronomia, Alimentos, Ambiente e Paisagem, Tapada da Ajuda, 1349-017 Lisboa, Portugal 4 Universidade Eduardo Mondlane, Faculdade de Agronomia e Engenharia Florestal, Av. Julius Nyerere, Campus 3453, Maputo, Moçambique Original Article ABSTRACT: Pterocarpus angolensis and Terminalia sericea are two African species with medicinal potential. Despite the importance of their bark as a *Corresponding author: powerful astringent to treat various diseases it is poor described. In order to [email protected] provide referential information for correct identification and standardization of the Keywords: plant material, bark samples from each two species were collected and analyzed Secondary phloem under light and electron microscopy. Some important anatomical features to identification were: the sclerenchyma tissue mostly in form of fibre-sclereids and Anatomical structure the large secretory cells arranged in conspicuous rows or tangential bands in the Species distinction conducting phloem in P. angolensis; the crystalliferous cells arranged in very regular tangential rows (druses) and the occurrence of large crystal cells near or Medicinal plants. including the tangential fibre bundles in T. sericea bark. The results obtained show Palavras-chave: that the anatomy of the bark can be used as an important subsidy in identification Floema secundário and standardization of the studied species contributing the scientific knowledge for more effective forms of scrutiny in preventing commercial adulteration of Estrutura anatômica species. -

Dicot/Monocot Root Anatomy the Figure Shown Below Is a Cross Section of the Herbaceous Dicot Root Ranunculus. the Vascular Tissu
Dicot/Monocot Root Anatomy The figure shown below is a cross section of the herbaceous dicot root Ranunculus. The vascular tissue is in the very center of the root. The ground tissue surrounding the vascular cylinder is the cortex. An epidermis surrounds the entire root. The central region of vascular tissue is termed the vascular cylinder. Note that the innermost layer of the cortex is stained red. This layer is the endodermis. The endodermis was derived from the ground meristem and is properly part of the cortex. All the tissues inside the endodermis were derived from procambium. Xylem fills the very middle of the vascular cylinder and its boundary is marked by ridges and valleys. The valleys are filled with phloem, and there are as many strands of phloem as there are ridges of the xylem. Note that each phloem strand has one enormous sieve tube member. Outside of this cylinder of xylem and phloem, located immediately below the endodermis, is a region of cells called the pericycle. These cells give rise to lateral roots and are also important in secondary growth. Label the tissue layers in the following figure of the cross section of a mature Ranunculus root below. 1 The figure shown below is that of the monocot Zea mays (corn). Note the differences between this and the dicot root shown above. 2 Note the sclerenchymized endodermis and epidermis. In some monocot roots the hypodermis (exodermis) is also heavily sclerenchymized. There are numerous xylem points rather than the 3-5 (occasionally up to 7) generally found in the dicot root. -

Eudicots Monocots Stems Embryos Roots Leaf Venation Pollen Flowers
Monocots Eudicots Embryos One cotyledon Two cotyledons Leaf venation Veins Veins usually parallel usually netlike Stems Vascular tissue Vascular tissue scattered usually arranged in ring Roots Root system usually Taproot (main root) fibrous (no main root) usually present Pollen Pollen grain with Pollen grain with one opening three openings Flowers Floral organs usually Floral organs usually in in multiples of three multiples of four or five © 2014 Pearson Education, Inc. 1 Reproductive shoot (flower) Apical bud Node Internode Apical bud Shoot Vegetative shoot system Blade Leaf Petiole Axillary bud Stem Taproot Lateral Root (branch) system roots © 2014 Pearson Education, Inc. 2 © 2014 Pearson Education, Inc. 3 Storage roots Pneumatophores “Strangling” aerial roots © 2014 Pearson Education, Inc. 4 Stolon Rhizome Root Rhizomes Stolons Tubers © 2014 Pearson Education, Inc. 5 Spines Tendrils Storage leaves Stem Reproductive leaves Storage leaves © 2014 Pearson Education, Inc. 6 Dermal tissue Ground tissue Vascular tissue © 2014 Pearson Education, Inc. 7 Parenchyma cells with chloroplasts (in Elodea leaf) 60 µm (LM) © 2014 Pearson Education, Inc. 8 Collenchyma cells (in Helianthus stem) (LM) 5 µm © 2014 Pearson Education, Inc. 9 5 µm Sclereid cells (in pear) (LM) 25 µm Cell wall Fiber cells (cross section from ash tree) (LM) © 2014 Pearson Education, Inc. 10 Vessel Tracheids 100 µm Pits Tracheids and vessels (colorized SEM) Perforation plate Vessel element Vessel elements, with perforated end walls Tracheids © 2014 Pearson Education, Inc. 11 Sieve-tube elements: 3 µm longitudinal view (LM) Sieve plate Sieve-tube element (left) and companion cell: Companion cross section (TEM) cells Sieve-tube elements Plasmodesma Sieve plate 30 µm Nucleus of companion cell 15 µm Sieve-tube elements: longitudinal view Sieve plate with pores (LM) © 2014 Pearson Education, Inc.