The Anatomy As a Tool for the Identification of the Bark of Pterocarpus Angolensis and Terminalia Sericea1 a Anatomia Como Ferra
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bark Anatomy and Cell Size Variation in Quercus Faginea
Turkish Journal of Botany Turk J Bot (2013) 37: 561-570 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1201-54 Bark anatomy and cell size variation in Quercus faginea 1,2, 2 2 2 Teresa QUILHÓ *, Vicelina SOUSA , Fatima TAVARES , Helena PEREIRA 1 Centre of Forests and Forest Products, Tropical Research Institute, Tapada da Ajuda, 1347-017 Lisbon, Portugal 2 Centre of Forestry Research, School of Agronomy, Technical University of Lisbon, Tapada da Ajuda, 1349-017 Lisbon, Portugal Received: 30.01.2012 Accepted: 27.09.2012 Published Online: 15.05.2013 Printed: 30.05.2013 Abstract: The bark structure of Quercus faginea Lam. in trees 30–60 years old grown in Portugal is described. The rhytidome consists of 3–5 sequential periderms alternating with secondary phloem. The phellem is composed of 2–5 layers of cells with thin suberised walls and narrow (1–3 seriate) tangential band of lignified thick-walled cells. The phelloderm is thin (2–3 seriate). Secondary phloem is formed by a few tangential bands of fibres alternating with bands of sieve elements and axial parenchyma. Formation of conspicuous sclereids and the dilatation growth (proliferation and enlargement of parenchyma cells) affect the bark structure. Fused phloem rays give rise to broad rays. Crystals and druses were mostly seen in dilated axial parenchyma cells. Bark thickness, sieve tube element length, and secondary phloem fibre wall thickness decreased with tree height. The sieve tube width did not follow any regular trend. In general, the fibre length had a small increase toward breast height, followed by a decrease towards the top. -

Tree Identification: from Bark and Leaves Or Needles
Tree Identification: from bark and leaves or needles A walk in the woods can be a lot of fun, especially if you bring your kids. How do you get them to come along with you? Tell them this. “Look at the bark on the trees. Can you can find any that look like burnt potato chips, warts, cat scratches, camouflage pants or rippling muscles?” Believe it or not, these are descriptions of different kinds of tree bark. Tree ID from bark:______________________________________________________ Black cherry: The bark looks like burnt potato chips. Hackberry: The bark is bumpy and warty. Ironwood: The bark has long thin strips. With a little imagination, an ironwood can look like it is used as a scratching post by cats. They can also be easily spotted in winter because their light brown dead leaves hang on well past the first snow. Sycamore: A tree with bark that looks like camouflaged pants. The largest tree in Illinois is a sycamore, a majestic 115-foot tree near Springfield. Musclewood: a tree with smooth gray bark covering a trunk with ridges that look like they are rippling muscles. Shagbark hickory: Long strips of shaggy bark peeling at both ends. Cottonwood: Has heavy ridges that make it look like Paul Bunyan’s corduroy pants. Bur oak: Thick and gnarly bark has deep craggy furrows. The corky bark allows it to easily withstand hot forest fires. Tree ID from leaves or needles:________________________________________ Willow Oak: Has elongated leaves similar to those of a willow tree. Red pine: Red has 3 letters; a red pine has groupings of 2-3 prickly needles. -
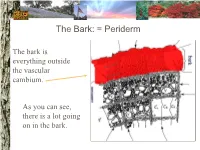
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division. -

Getting Chemicals Into Trees Without Spraying
Urban Forestry NR/FF/020 (pr) Getting Chemicals Into Trees Without Spraying Michael Kuhns, Forestry Extension Specialist This fact sheet provides an overview of injection, or phellogen that makes cork to thicken the outer bark, implantation, and other ways to get chemicals, mainly phloem that conducts food through the tree from where pesticides, into trees. Many techniques and systems it is stored or made to where it is being used (all of these exist and some are very good, some are good in some tissues together make up the bark), vascular cambium situations, and some are ineffective or bad for trees. that divides rapidly to make new phloem and xylem cells, This fact sheet addresses all of these. and xylem or wood. Xylem includes an outer layer called the sapwood that conducts mostly water and minerals from the roots to the canopy, and an inner layer called the Chemicals are applied to trees for many reasons. heartwood that is aged sapwood that has died and has lost Insecticides repel or kill damaging insects, fungicides its ability to conduct water but still adds strength. treat or prevent fungal diseases, nutrients and plant growth regulators affect growth, and herbicides kill trees or prevent sprouting after tree removal. Spraying is the most typical way to apply these chemicals. It is fast, uses readily available equipment, and is understood. The Phellem (Cork Phellogen down side of spraying is that much of the chemical being or Outer Bark) Phloem applied is wasted, either to drift, run off, or because it can not be applied precisely to where it is needed in the tree. -

Tree Anatomy Stems and Branches
Tree Anatomy Series WSFNR14-13 Nov. 2014 COMPONENTSCOMPONENTS OFOF PERIDERMPERIDERM by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Around tree roots, stems and branches is a complex tissue. This exterior tissue is the environmental face of a tree open to all sorts of site vulgarities. This most exterior of tissue provides trees with a measure of protection from a dry, oxidative, heat and cold extreme, sunlight drenched, injury ridden site. The exterior of a tree is both an ecological super highway and battle ground – comfort and terror. This exterior is unique in its attributes, development, and regeneration. Generically, this tissue surrounding a tree stem, branch and root is loosely called bark. The tissues of a tree, outside or more exterior to the xylem-containing core, are varied and complexly interwoven in a relatively small space. People tend to see and appreciate the volume and physical structure of tree wood and dismiss the remainder of stem, branch and root. In reality, tree life is focused within these more exterior thin tissue sets. Outside of the cambium are tissues which include transport cells, structural support cells, generation cells, and cells positioned to help, protect, and sustain other cells. All of this life is smeared over the circumference of a predominately dead physical structure. Outer Skin Periderm (jargon and antiquated term = bark) is the most external of tree tissues providing protection, water conservation, insulation, and environmental sensing. Periderm is a protective tissue generated over and beyond live conducting and non-conducting cells of the food transport system (phloem). -

Why Do Animals Eat the Bark and Wood of Trees and Shrubs? William R
FNR-203 Purdue University & Natural Re ry sou Forestry and Natural Resources st rc re e o s F Why Do Animals Eat the Bark and Wood of Trees and Shrubs? William R. Chaney, Professor of Tree Physiology Department of Forestry and Natural Resources Purdue University, West Lafayette, IN 47907 PURDUE UNIVERSITY Animals gnawing the bark and wood of trees anatomy, movement and storage of food in trees, and shrubs is not a malicious act or evidence of a and the variations in digestive systems among neurotic condition. Instead, it is the normal means animals. by which some animals acquire a nutritious food source. The ability to consume this seemingly Constituents of Plant Cell Walls unpalatable food supply and derive nourishment Unlike animals, plants have cells with rigid from it requires specialized feeding habits and cell walls composed of cellulose, hemicellulose, digestive systems. and lignins. Pectins help hold the individual cells together in tissues. All of these substances are potential sources of food for animals. Cellulose The most consummate wood feeders are the A principal component of cell walls is billions of termites throughout the world that cellulose, which consists of 5,000 to 10,000 literally devour thousands of tons of woody glucose molecules joined by a so-called beta debris every year in forests as well as lumber linkage to form straight chain polymers of pure in buildings. Many of our most serious and sugar. The long chains of cellulose are combined damaging insect pests are the bark beetles and first into microfibrils and then further into wood borers that feed on the parts of trees for macrofibrils to create a mesh-like matrix of cell which they are named. -

Xylem Resin in the Resistance of the Pinaceae to Bark Beetles
XYLEM RESIN IN THE RESISTANCE OF THE PINACEAE TO BARK BEETLES Richard H. Smith Xylem resin-a supersaturation of resin acids in terpenes- appears to play a paradoxical role in the relation of the Pinaceae to tree-killing bark beetles. It has been suggested as the agent responsible for the susceptibility of this coniferous family to beetle attacks. And, at the same time, it has been linked with the ability of the Pinaceae to resist bark beetles. The hosts of tree- killing bark beetles are nearly all Pinaceae-Pinus (pine), Abies (fir), Pseudotsuga (Douglas-fir), Picea (spruce), Tsuga (hemlock), and Larix (larch). Significantly, among conifers, xylem resin is most common and abundant in these Pinaceae. Bark beetles are found on other families of conifers, but they are usually con- sidered of minor importance. Much experimental work has been done since the early reports of an apparent association of resin with resistance. Nearly all studies have dealt with attacking adult beetles; virtually no re- search has been directed at effects of resin on immature forms. Most work has been with Pinus and Dep'roctonus, with lesser attention to spruce, fir, Douglas-fir, and the other genera of bark beetles. PACIFIC This report summarizes the early findings, updates them with results of more recent reports and, in some cases, reinterprets SOUTHWEST these previous reports. This review deals only with bark beetles that attack living trees and with xylem resin, although in a few Forest and Ranee instances, it concerns resin-related chemicals as well. This review follows the approach of Painter (1951), who pro- Experiment station posed that plant resistance to insects depends on one or more FOREST SERVICE U. -

Dicot/Monocot Root Anatomy the Figure Shown Below Is a Cross Section of the Herbaceous Dicot Root Ranunculus. the Vascular Tissu
Dicot/Monocot Root Anatomy The figure shown below is a cross section of the herbaceous dicot root Ranunculus. The vascular tissue is in the very center of the root. The ground tissue surrounding the vascular cylinder is the cortex. An epidermis surrounds the entire root. The central region of vascular tissue is termed the vascular cylinder. Note that the innermost layer of the cortex is stained red. This layer is the endodermis. The endodermis was derived from the ground meristem and is properly part of the cortex. All the tissues inside the endodermis were derived from procambium. Xylem fills the very middle of the vascular cylinder and its boundary is marked by ridges and valleys. The valleys are filled with phloem, and there are as many strands of phloem as there are ridges of the xylem. Note that each phloem strand has one enormous sieve tube member. Outside of this cylinder of xylem and phloem, located immediately below the endodermis, is a region of cells called the pericycle. These cells give rise to lateral roots and are also important in secondary growth. Label the tissue layers in the following figure of the cross section of a mature Ranunculus root below. 1 The figure shown below is that of the monocot Zea mays (corn). Note the differences between this and the dicot root shown above. 2 Note the sclerenchymized endodermis and epidermis. In some monocot roots the hypodermis (exodermis) is also heavily sclerenchymized. There are numerous xylem points rather than the 3-5 (occasionally up to 7) generally found in the dicot root. -

Eudicots Monocots Stems Embryos Roots Leaf Venation Pollen Flowers
Monocots Eudicots Embryos One cotyledon Two cotyledons Leaf venation Veins Veins usually parallel usually netlike Stems Vascular tissue Vascular tissue scattered usually arranged in ring Roots Root system usually Taproot (main root) fibrous (no main root) usually present Pollen Pollen grain with Pollen grain with one opening three openings Flowers Floral organs usually Floral organs usually in in multiples of three multiples of four or five © 2014 Pearson Education, Inc. 1 Reproductive shoot (flower) Apical bud Node Internode Apical bud Shoot Vegetative shoot system Blade Leaf Petiole Axillary bud Stem Taproot Lateral Root (branch) system roots © 2014 Pearson Education, Inc. 2 © 2014 Pearson Education, Inc. 3 Storage roots Pneumatophores “Strangling” aerial roots © 2014 Pearson Education, Inc. 4 Stolon Rhizome Root Rhizomes Stolons Tubers © 2014 Pearson Education, Inc. 5 Spines Tendrils Storage leaves Stem Reproductive leaves Storage leaves © 2014 Pearson Education, Inc. 6 Dermal tissue Ground tissue Vascular tissue © 2014 Pearson Education, Inc. 7 Parenchyma cells with chloroplasts (in Elodea leaf) 60 µm (LM) © 2014 Pearson Education, Inc. 8 Collenchyma cells (in Helianthus stem) (LM) 5 µm © 2014 Pearson Education, Inc. 9 5 µm Sclereid cells (in pear) (LM) 25 µm Cell wall Fiber cells (cross section from ash tree) (LM) © 2014 Pearson Education, Inc. 10 Vessel Tracheids 100 µm Pits Tracheids and vessels (colorized SEM) Perforation plate Vessel element Vessel elements, with perforated end walls Tracheids © 2014 Pearson Education, Inc. 11 Sieve-tube elements: 3 µm longitudinal view (LM) Sieve plate Sieve-tube element (left) and companion cell: Companion cross section (TEM) cells Sieve-tube elements Plasmodesma Sieve plate 30 µm Nucleus of companion cell 15 µm Sieve-tube elements: longitudinal view Sieve plate with pores (LM) © 2014 Pearson Education, Inc. -

An Overview of Properties of Cork – a Bottling Approach
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395 -0056 Volume: 03 Issue: 04 | Apr -2016 www.irjet.net p-ISSN: 2395-0072 AN OVERVIEW OF PROPERTIES OF CORK – A BOTTLING APPROACH Akshaykumar M. Shinde1, Ganesh J. Tate2, Rohit R. Shinde3, Sagar P. Kadam4, Pranit M. Patil5 1, 2, 3,4 BE Students, Department of Mechanical Engineering, GIT, Lavel (MH), India 5 Assistant Professor & Guide, Department of Mechanical Engineering, GIT, Lavel (MH), India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract - Cork is a material which has been used for Cork physical-mechanical characteristics make it an multiple applications. The most known uses of cork are excellent material for thermal insulation, most advantageously, e.g., in cold chambers where compressive in stoppers (natural and agglomerated cork) for loads are present, and also for acoustic absorption (e.g., alcoholic beverages, classic floor covering with recording studios) and vibration insulation (e.g., composite cork tiles (made by the binding of cork machinery). Its pleasant sensation to the touch, energy particles with different binders), Cork is a natural, absorbing and anti-sliding properties make it also good for renewable, sustainable raw material that has been coverings, shoes or in handles. Its compression-recovery used for many centuries. As a result of this very long properties make it the material of choice for seals and joints in civil construction, woodwind instruments and term interest, the scientific literature on cork is combustion engines and, of course, as stoppers [1,2]. extensive. Cork is resilient, strong (high specific strength), impervious to water, has a near zero Poisson 2. TYPES OF CLOSURES coefficient, very low thermal conductivity, low density There are different types of closures used in bottling and a complex chemical structure. -

Bark Structure of North American Conifers
2 5 1.0 :: 11111 2.8 11111 . W ~~~ W a.:.: Ii.£ I"~Q ~"I~ IIIP'~ 1.1 III.~ ".. ~~L8 ·1111'1.25 111111.4 111111.6 1111,1.25 111111.4 111111.6 MICROCOPY RESOLUTION TEST CHART MICROCOPY RESOLUTION TEST CHART NATIONAL BUREAU O~ STANDARDS-1963-A NATIONAL BUREAU OF STANDARDS-1963-A CONTENTS Page ·,Introduction_____________ _ . _______ _ 1 .Mllterial and methods_. __ .. _____ _ 2 --MateriaL_______ ' __ •. _._ 2 __ ,Methods___ . __ _ ... 4 "11 Description of bark structure and Rpccific and gcncric characteristics_ 8 Taxaceac_ _ ___ . _____ .. _. _. ... __ 8 Pinaceae___ ___ _ _ . __ _ _ . _ 12 Diagnostic featurcs of bark and their applicatioll to idcntificatioll__ 67 __ Artificial keys to familics and gcncra__ _ _____ ._.___ . 67 Notes on random chcmicaimeans fnr idcntificatioll. __ _ 74 Discussion__ • __ . __ __ _ __ _ _ _ __ __ __._ __ _ _ __ _ __ __ ________ __ Y5 Significance of coniferous bark structurc_. .... i'5 Correlation of conifcrous bark structure to wood slructuJ'{' __ _ irs Relation of bark structure to other rcsearch _________________ . Summary_______________________ ..._ 8~; 83 Lit.erature cited____ _ 84 II "Por snlc by the Sup~rint~n'l.ullt nf Documents, U. S. Go\'crnlll~nt Printing Omce • WflShillgt,on 25, D. C. -- Prke:lS cents .S:.tiuct{.re of North ...... t J; Americall Conifers 1 '. By YIXG-PE OH...~'G,: collaboratol', Forest ProcZllcts Laboratory; Forest Serr:ice INTRODUCTION ~.\.n anatomical description of bark cells was first recorded in the 17th century, when Robert Hooke observed "cells" in cork and other tissues Undel: the "mafrnifyin~ lens" and published his discovery in 1665 in his ~1icrographia, Founders of plant anatomy. -

How a Tree Works!
How A Tree Works! Essential Question(s): Location: Indoors or outdoors How does a tree work? Objectives: Learners will 1) describe the parts of a At a Glance: In this activity, learners will learn about different parts tree. of a tree and how they function as an entire system by building a 2) explain how each part “human” tree. Learners will learn how food is made and stored as an functions or works. introduction to photosynthesis. Skills: communication, listening, analysis Background Information: Tree Parts and How They “Work” Supplies: Although there are thousands of different kinds of trees in the world, slips of paper with the most trees work the same way. Here's a look at how the parts of a tree names of tree parts written work together to help a tree get the food, water, and minerals it needs on them to survive. cross section of a tree 1. The Trunk: The trunk of a tree is important for two reasons: First, diagram tree “cookies” (cross it acts as a support rod, giving the tree its shape and strength. Second, sections of a tree) it acts as the central "plumbing system" in a tree, forming a network of tubes that carries water and minerals up from the roots to the Subjects: language arts, leaves, and food (sugar) from the leaves down to the branches, trunk, science and roots. The easiest way to see how a tree works is to look at a cross section of the trunk. Look at the diagram to see the five main Time: 20 minutes layers and what each layer does.