Geographic Assignment of Seabirds to Their Origin: Combining Morphologic, Genetic, and Biogeochemical Analyses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systematic List of the Birds
59.82(66.53) Article III.-THE MARINE ORNITHOLOGY OF THE CAPE VERDE ISLANDS, WITH A LIST OF ALL THE BIRDS OF THE ARCHIPELAGO BY ROBERT CUSHMAN MURPHY CONTENTS PAGE. INTRODUCTION........................................................... 211 HISTORICAL SKETCH OF CAPE VERDE ORNITHOLOGY .............. .......... 212 THE VISITS OF CORREIA AND THE AUTHOR.................................. 213 THE GEOGRAPHIC ENVIRONMENT....................... 216 OCEANOGRAPHY AND CLIMATE............................................ 221 GEOGRAPHIC RELATIONSHIPS OF THE BIRDS ............................... 226 BIBLIOGRAPHY......................................................... 232 SYSTEMATIC LIST OF THE BIRDS............................................ 233 INTRODUCTION The present paper is an indirect result of a brief visit which the writer made to the Cape Verde Islands in September 1912. It is more immediately linked, however, with a collecting trip undertaken during part of the spring and summer of 1922 by Mr. Jos6 Gongalves Correia, of New Bedford, Mass., whose specimens, numbering some three hundred skins of twenty species, have all been received by The American Museum of Natural History. As Correia's predominant object was to acquire adequate series of water birds, the terrestrial species of the Cape Verdes are but scantily represented in his collection, which is supplemented, to a certain extent, by a small number of specimens obtained by the writer in 1912. Notwithstanding the relatively limited material available for study, it has been thought advisable to include within this paper a systematic list of all the birds known from the Cape Verde Islands. The latest faunal contribution is that of Salvadori (1899). Not only have addi- tions to the avifauna been made since that date, but also forms new to science, including several endemic races, have been described. More- over, numerous nomenclatural changes made necessary because of the researches of Hartert ('V6gel der palaarktischen Fauna,' 1910-22) and other workers increase the need for a new list. -

Onetouch 4.0 Scanned Documents
/ Chapter 2 THE FOSSIL RECORD OF BIRDS Storrs L. Olson Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution Washington, DC. I. Introduction 80 II. Archaeopteryx 85 III. Early Cretaceous Birds 87 IV. Hesperornithiformes 89 V. Ichthyornithiformes 91 VI. Other Mesozojc Birds 92 VII. Paleognathous Birds 96 A. The Problem of the Origins of Paleognathous Birds 96 B. The Fossil Record of Paleognathous Birds 104 VIII. The "Basal" Land Bird Assemblage 107 A. Opisthocomidae 109 B. Musophagidae 109 C. Cuculidae HO D. Falconidae HI E. Sagittariidae 112 F. Accipitridae 112 G. Pandionidae 114 H. Galliformes 114 1. Family Incertae Sedis Turnicidae 119 J. Columbiformes 119 K. Psittaciforines 120 L. Family Incertae Sedis Zygodactylidae 121 IX. The "Higher" Land Bird Assemblage 122 A. Coliiformes 124 B. Coraciiformes (Including Trogonidae and Galbulae) 124 C. Strigiformes 129 D. Caprimulgiformes 132 E. Apodiformes 134 F. Family Incertae Sedis Trochilidae 135 G. Order Incertae Sedis Bucerotiformes (Including Upupae) 136 H. Piciformes 138 I. Passeriformes 139 X. The Water Bird Assemblage 141 A. Gruiformes 142 B. Family Incertae Sedis Ardeidae 165 79 Avian Biology, Vol. Vlll ISBN 0-12-249408-3 80 STORES L. OLSON C. Family Incertae Sedis Podicipedidae 168 D. Charadriiformes 169 E. Anseriformes 186 F. Ciconiiformes 188 G. Pelecaniformes 192 H. Procellariiformes 208 I. Gaviiformes 212 J. Sphenisciformes 217 XI. Conclusion 217 References 218 I. Introduction Avian paleontology has long been a poor stepsister to its mammalian counterpart, a fact that may be attributed in some measure to an insufRcien- cy of qualified workers and to the absence in birds of heterodont teeth, on which the greater proportion of the fossil record of mammals is founded. -

FEEDING ECOLOGY of the CAPE VERDEAN SHEARWATER (Calonectris Edwardsii) POPULATION of RASO ISLET, CAPE VERDE (P1-B-11)
2nd World Seabird Conference “Seabirds: Global Ocean Sentinels” 26-30 October 2015 Cape Town, South Africa FEEDING ECOLOGY OF THE CAPE VERDEAN SHEARWATER (Calonectris edwardsii) POPULATION OF RASO ISLET, CAPE VERDE (P1-B-11) Isabel Rodrigues1,3; Nuno Oliveira 2 Rui Freitas 3 Tommy Melo1 Pedro Geraldes 2 1Biosfera I, Cabo Verde, www.biosfera1.com; 2SPEA, Portugal, www.spea.pt, 3 Universidade de Cabo Verde, www.unicv.edu.cv Raso Islet with only 5.76 km2, is an area of great importance to Cape Verdean Shearwaters as we find one of the largest colonies of the species there. The great biological value of this islet is even more remarkable for hosting very large populations of other species, such as the Brown Booby, Red-billed Tropicalbird and even the endemic Raso Lark, among others. Along with Branco Islet, also included in the Nature Reserve, both populations constitute about 75% of the nesting population of the Cape Verde islands (Fig. 1). The Cape Verde Shearwater (Procellariiformes, Procellariidae) (Fig. 2) is an endemic species of Cape Verde and has recently been separated from Calonectris diomedea species, due to their morphological and genetic differences, and pelagic habits; feeding mostly on the open sea (Hazevoet 1995). METHODOLOGY RESULTS The samples were collected between 14 October and 12 November, in two In total, 80 regurgitations from juvenile Cape Verde Shearwaters were collected; consecutive years, 2012 and 2013. We randomly obtained 80 samples of juvenile including 50 individuals sampled in 2012, and 30 individuals in 2013. regurgitation. Each juvenile was sampled only once. During or after handling, Based on knowledge of local fish populations and according to the current juveniles tend to regurgitate stomach contents without the need to resort to the description, the identified prey of Cape Verde shearwaters belonged to 5 species induced regurgitation method. -

ORIGIN and EVOLUTION of BIRDS Dr. Ramesh Pathak B.Sc. (Hons.) –II
ORIGIN AND EVOLUTION OF BIRDS Dr. Ramesh Pathak B.Sc. (hons.) –II Prof. Parker has shown a number of peculiarities between birds and repiles,so,he said “birds are transformed and glorified reptiles”. Huxley has established a very close relationship by saying birds are “feathered reptiles”. THEORIES OF ORIGIN OF BIRDS A. Cursorial theory : This theory was championed by Baron Nopsa who maintained that the birds evolved from cursorial bipedal dinosaurs. In attempt to move faster during running to pull themselves a bit faster they moved and beat their arms. In such condition, due to continuous use of arms as propellers brought constant increase in the length and breadth of scales present on them. As the scales lengthened ,the pressure against the air caused their edges frayed leading to scales changing into feathers. But this theory was rejected because scales and feathers are fundamentally different structures arising from different layers of skin. B.Tetrapteryx theory :It was proposed by Beebe and Gregorg. According to this theory the ancestors of birds were arboreal reptiles who used to jump from branch to branch and in doing so the scales were transformed into feathers by fraying of the edges. After developing feathers on all the four limbs (Tetrapteryx stage), they used only the anterior wings and the unused posterior wings were lost. This theory was also discarded because there is no evidence that birds had ever four wings. C. Gliding thery : It is based on the idea of Beebe and Georg.It was proposed by Heilmann but does not recognize the tetrapteryx stage. -

Print BB May 2005
Weather and bird migration Norman Elkins Richard Johnson ABSTRACT Bird migration in and through Britain and Ireland shows great variation, both seasonally and annually. Much depends on atmospheric conditions, which can equally hinder and assist migration, both on a broad scale (the distribution, extent, intensity and movement of pressure patterns), and at a local level (temporal and spatial changes in wind, visibility, cloud and precipitation).All the parameters of bird migration, including timing, routes, speed and duration of migration, are affected, and modified, by a number of meteorological factors, probably the most influential being wind speed and direction.This paper reviews the influence that weather has on bird migration in Britain and Ireland, and discusses examples of the major types of migration. A number of unusual or exceptional migration events are discussed and illustrated with appropriate weather charts. Introduction long-distance expeditions traversing half the Migration is one topic that never fails to appeal planet, such as those by Arctic Terns Sterna par- to the whole spectrum of those with an interest adisaea and Barn Swallows Hirundo rustica,to in birds, from the inveterate ‘twitcher’ to the local movements in response to the temporary general public. Bird migration, defined simply freezing of freshwater bodies. Differences in as regular movements from one area to another, migration strategy and behaviour result in generally occurs in response to the availability some species undertaking nocturnal migration, of food resources and encompasses an enor- while others move mainly during daylight mous variety of methods. These range from hours. These differences operate not only 238 © British Birds 98 • May 2005 • 238-256 126. -

Paravian Phylogeny and the Dinosaur-Bird Transition: an Overview
feart-06-00252 February 11, 2019 Time: 17:42 # 1 REVIEW published: 12 February 2019 doi: 10.3389/feart.2018.00252 Paravian Phylogeny and the Dinosaur-Bird Transition: An Overview Federico L. Agnolin1,2,3*, Matias J. Motta1,3, Federico Brissón Egli1,3, Gastón Lo Coco1,3 and Fernando E. Novas1,3 1 Laboratorio de Anatomía Comparada y Evolución de los Vertebrados, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina, 2 Fundación de Historia Natural Félix de Azara, Universidad Maimónides, Buenos Aires, Argentina, 3 Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina Recent years witnessed the discovery of a great diversity of early birds as well as closely related non-avian theropods, which modified previous conceptions about the origin of birds and their flight. We here present a review of the taxonomic composition and main anatomical characteristics of those theropod families closely related with early birds, with the aim of analyzing and discussing the main competing hypotheses pertaining to avian origins. We reject the postulated troodontid affinities of anchiornithines, and the Edited by: dromaeosaurid affinities of microraptorians and unenlagiids, and instead place these Corwin Sullivan, University of Alberta, Canada groups as successive sister taxa to Avialae. Aiming to evaluate previous phylogenetic Reviewed by: analyses, we recoded unenlagiids in the traditional TWiG data matrix, which resulted Thomas Alexander Dececchi, in a large polytomy at the base of Pennaraptora. This indicates that the TWiG University of Pittsburgh, United States phylogenetic scheme needs a deep revision. Regarding character evolution, we found Spencer G. Lucas, New Mexico Museum of Natural that: (1) the presence of an ossified sternum goes hand in hand with that of ossified History & Science, United States uncinate processes; (2) the presence of foldable forelimbs in basal archosaurs indicates *Correspondence: widespread distribution of this trait among reptiles, contradicting previous proposals Federico L. -

On the Origin and Evolution of Nest Building by Passerine Birds’
T H E C 0 N D 0 R r : : ,‘ “; i‘ . .. \ :i A JOURNAL OF AVIAN BIOLOGY ,I : Volume 99 Number 2 ’ I _ pg$$ij ,- The Condor 99~253-270 D The Cooper Ornithological Society 1997 ON THE ORIGIN AND EVOLUTION OF NEST BUILDING BY PASSERINE BIRDS’ NICHOLAS E. COLLIAS Departmentof Biology, Universityof California, Los Angeles, CA 90024-1606 Abstract. The object of this review is to relate nest-buildingbehavior to the origin and early evolution of passerinebirds (Order Passeriformes).I present evidence for the hypoth- esis that the combinationof small body size and the ability to place a constructednest where the bird chooses,helped make possiblea vast amountof adaptiveradiation. A great diversity of potential habitats especially accessibleto small birds was created in the late Tertiary by global climatic changes and by the continuing great evolutionary expansion of flowering plants and insects.Cavity or hole nests(in ground or tree), open-cupnests (outside of holes), and domed nests (with a constructedroof) were all present very early in evolution of the Passeriformes,as indicated by the presenceof all three of these basic nest types among the most primitive families of living passerinebirds. Secondary specializationsof these basic nest types are illustratedin the largest and most successfulfamilies of suboscinebirds. Nest site and nest form and structureoften help characterizethe genus, as is exemplified in the suboscinesby the ovenbirds(Furnariidae), a large family that builds among the most diverse nests of any family of birds. The domed nest is much more common among passerinesthan in non-passerines,and it is especially frequent among the very smallestpasserine birds the world over. -

Recent Bird Records from Fogo, Cape Verde Islands Rubén Baronea and Jens Heringb
Recent bird records from Fogo, Cape Verde Islands Rubén Baronea and Jens Heringb Observations récentes de Fogo, Îles du Cap-Vert. Des données sont présentées concernant 12 espèces d’oiseaux observées à Fogo, Îles du Cap-Vert, parmi lesquelles deux premières mentions pour l’île (Chevalier gambette Tringa totanus et Hirondelle de fenêtre Delichon urbicum), les premières données de nidification du Martinet du Cap-Vert Apus alexandri et les premières observations fiables du Phaéton à bec rouge Phaethon aethereus indiquant la nidification probable de celui-ci. Des informations sont également présentées sur d’autres taxons mal connus à Fogo, tels que certaines espèces pélagiques et l’Effraie des clochers Tyto alba detorta. Summary. We present data on 12 bird species observed on Fogo, Cape Verde Islands, among them two first records for the island (Common Redshank Tringa totanus and Common House Martin Delichon urbicum), the first breeding records of Cape Verde Swift Apus alexandri and the first reliable observations of Red-billed Tropicbird Phaethon aethereus indicating probable breeding. Information on other taxa poorly known on Fogo, such as some pelagic seabirds and Barn Owl Tyto alba detorta, is also given. ogo, one of the Cape Verde Islands, is situated reported previously, and some others for which F in the leeward group (‘Ilhas do Sotavento’), there are only a limited number of observations. c.724 km from the African continent. With Local information on breeding birds was mainly a surface area of 478 km2, the highest peak collected by RB. Dates of our visits are as follows: (Pico Novo) reaches 2,829 m (Michell-Thomé 18–21 October 2004 (JH & H. -
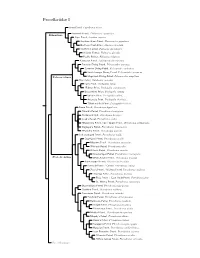
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Interpreting Behavior from Early Cretaceous Bird Tracks and the Morphology of Bird Feet and Trackways
INTERPRETING BEHAVIOR FROM EARLY CRETACEOUS BIRD TRACKS AND THE MORPHOLOGY OF BIRD FEET AND TRACKWAYS By ©2009 Amanda Renee Falk B.S., Lake Superior State University, 2007 Submitted to the Department of Geology and the Faculty of the Graduate School of the University of Kansas In partial fulfillment of the requirements for the degree of Master of Science Advisory Committee: ______________________________ Co-Chairman: Stephen T. Hasiotis ______________________________ Co-Chairman: Larry D. Martin ______________________________ J. F. Devlin Date Defended: September 15th, 2009 The thesis committee for Amanda R. Falk certifies that this is the approved version of the following thesis: INTERPRETING BEHAVIOR FROM EARLY CRETACEOUS BIRD TRACKS AND THE MORPHOLOGY OF BIRD FEET AND TRACKWAYS Advisory Committee: ____________________________ Stephen T. Hasiotis, Chairman ____________________________ Larry D. Martin, Co-Chairman ____________________________ J. F. Devlin Date approved: _ September 15th, 2009_ ii ABSTRACT Amanda R. Falk Department of Geology, September 2009 University of Kansas Bird tracks were studied from the Lower Cretaceous Lakota Formation in South Dakota, USA, and the Lower Cretaceous Haman Formation, South Korea. Behaviors documented from the Lakota Formation included: (1) a takeoff behavior represented by a trackway terminating in two subparallel tracks; (2) circular walking; and (3) the courtship display high stepping. Behaviors documented from the Haman Formation included: (1) a low-angle landing in which the hallux toe was dragged; (2) pecking and probing behaviors; and (3) flapping-assisted hopping during walking. The invertebrate trace fossil Cochlichnus was associated the avian tracks from the Lakota Formation. No traces of pecking or probing were associated with Cochlichnus. The invertebrate trace fossils Cochlichnus, Arenicholites, and Steinichnus were found associated the bird tracks from the Haman Formation. -

The Origin and Diversification of Birds
Current Biology Review The Origin and Diversification of Birds Stephen L. Brusatte1,*, Jingmai K. O’Connor2,*, and Erich D. Jarvis3,4,* 1School of GeoSciences, University of Edinburgh, Grant Institute, King’s Buildings, James Hutton Road, Edinburgh EH9 3FE, UK 2Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China 3Department of Neurobiology, Duke University Medical Center, Durham, NC 27710, USA 4Howard Hughes Medical Institute, Chevy Chase, MD 20815, USA *Correspondence: [email protected] (S.L.B.), [email protected] (J.K.O.), [email protected] (E.D.J.) http://dx.doi.org/10.1016/j.cub.2015.08.003 Birds are one of the most recognizable and diverse groups of modern vertebrates. Over the past two de- cades, a wealth of new fossil discoveries and phylogenetic and macroevolutionary studies has transformed our understanding of how birds originated and became so successful. Birds evolved from theropod dino- saurs during the Jurassic (around 165–150 million years ago) and their classic small, lightweight, feathered, and winged body plan was pieced together gradually over tens of millions of years of evolution rather than in one burst of innovation. Early birds diversified throughout the Jurassic and Cretaceous, becoming capable fliers with supercharged growth rates, but were decimated at the end-Cretaceous extinction alongside their close dinosaurian relatives. After the mass extinction, modern birds (members of the avian crown group) explosively diversified, culminating in more than 10,000 species distributed worldwide today. Introduction dinosaurs Dromaeosaurus albertensis or Troodon formosus.This Birds are one of the most conspicuous groups of animals in the clade includes all living birds and extinct taxa, such as Archaeop- modern world. -

Shearwatersrefs V1.10.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2018 & 2019 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Ashley Fisher (www.scillypelagics.com) for the cover images. All images © the photographer. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.10 (January 2018). Cover Main image: Great Shearwater. At sea 3’ SW of the Bishop Rock, Isles of Scilly. 8th August 2009. Picture by Ashley Fisher. Vignette: Sooty Shearwater. At sea off the Isles of Scilly. 14th August 2009. Picture by Ashley Fisher. Species Page No. Audubon's Shearwater [Puffinus lherminieri] 34 Balearic Shearwater [Puffinus mauretanicus] 28 Bannerman's Shearwater [Puffinus bannermani] 37 Barolo Shearwater [Puffinus baroli] 38 Black-vented Shearwater [Puffinus opisthomelas] 30 Boyd's Shearwater [Puffinus boydi] 38 Bryan's Shearwater [Puffinus bryani] 29 Buller's Shearwater [Ardenna bulleri] 15 Cape Verde Shearwater [Calonectris edwardsii] 12 Christmas Island Shearwater [Puffinus nativitatis] 23 Cory's Shearwater [Calonectris borealis] 9 Flesh-footed Shearwater [Ardenna carneipes] 21 Fluttering