Mass Transfer and Hydrogenation of Acetone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) 11) 4,219,685 Savini 45 Aug
United States Patent (19) 11) 4,219,685 Savini 45 Aug. 26, 1980 (54) IMPROVING ODOR OF ISOPROPANOL Attorney, Agent, or Firm-C. Leon Kim; Rebecca Yablonsky 75 Inventor: Charles Savini, Warren, N.J. 57 ABSTRACT 73 Assignee: Exxon Research & Engineering Co., Methods for deodorizing lower alcohols such as ethanol Florham Park, N.J. and isopropyl alcohol and their oxy derivatives such as ethers and esters are disclosed, including contacting 21 Appl. No.: 31,761 these compounds with a deodorizing contact mass com 22 Filed: Apr. 20, 1979 prising, on a support, metals and/or metal oxides, pref erably of the metals of Group IB, VIB and VIII of the Periodic Table, where the metal oxides are at least par Related U.S. Application Data tially reduced to metal and the deodorizing contact 63 Continuation-in-part of Ser. No. 908,910, May 24, mass has a minimum particle dimension of greater than 1978, abandoned, which is a continuation-in-part of about 0.254 mm so as to be in a form suitable for use in Ser. No. 834,240, Sep. 19, 1977, abandoned. a fixed bed contacting process. In a preferred embodi ment, isopropyl alcohol is deodorized employing such 51 Int. Cl’.............................................. CO7C 29/24 deodorizing contact masses, preferably comprising 52 U.S. Cl. .............. ... 568/917; 568/922 Group VIII metals such as nickel, iron, cobalt and the 58) Field of Search ................................ 568/917,922 like, on a support, so that the contact mass has a surface 56) References Cited area less than about 1,500 m2 per gram. -

Transfer Hydrogenation of Olefins Catalysed by Nickel Nanoparticles
View metadata, citation and similar papers at core.ac.uk brought to you by CORE ARTICLE IN PRESS TET19911_proofprovided by Repositorio 26 October Institucional 2009 de la Universidad 1/7 de Alicante Tetrahedron xxx (2009) 1–7 Contents lists available at ScienceDirect Tetrahedron journal homepage: www.elsevier.com/locate/tet 55 1 56 2 Transfer hydrogenation of olefins catalysed by nickel nanoparticles 57 3 58 4 59 ** * 5 Francisco Alonso , Paola Riente, Miguel Yus 60 6 Departamento de Quı´mica Orga´nica, Facultad de Ciencias and Instituto de Sı´ntesis Orga´nica (ISO), Universidad de Alicante, Apdo. 99, 03080 Alicante, Spain 61 7 62 8 63 9 article info abstract 64 10 65 11 Article history: Nickel nanoparticles have been found to effectively catalyse the hydrogen-transfer reduction of a variety 66 12 Received 22 September 2009 of non-functionalised and functionalised olefins using 2-propanol as the hydrogen donor. The hetero- 67 13 Received in revised form geneous process has been shown to be highly chemoselective for certain substrates, with all the cor- 68 14 October 2009 responding alkanes being obtained in high yields. A synthesis of the natural dihydrostilbene brittonin 69 14 Accepted 15 October 2009 A is also reported based on the use of nickel nanoparticles. 15 Available online xxx 70 16 Ó 2009 Published by Elsevier Ltd. 71 17 Keywords: PROOF 72 18 Hydrogen transfer 73 19 Reduction 74 Olefins 75 20 Nickel nanoparticles 21 76 22 77 23 78 24 79 25 1. Introduction of chiral ligands. In contrast with the reduction of carbonyl com- 80 26 pounds, the hydrogen-transfer reduction of olefins has been little 81 27 The reduction of carbon–carbon double bonds is one of the studied, mainly involving noble-metal catalysts. -

Effect of Preparation Conditions of Raney Nickel on Its Catalytic Properties for Slurry Phase Hydrogenation of O-Nitrophenol to O-Aminophenol
Indian Journal of Chemical Technology Vol. 5, July 1998, pp. 199-208 Effect of preparation conditions of Raney Nickel on its catalytic properties for slurry phase hydrogenation of o-nitrophenol to o-aminophenol V R Choudhary'& M G Sane Chemical Engineering Division, National Chemical Laboratory, Pune 411 008, India Received 5 November 1997; accepted 10 June 1998 Influence of leaching conditions of Ni-Al alloy (50% AI) on the catalytic activity of Raney Nt (m the hydrogenation of o-nitrophenol at 308 K and H2-pressure of 1508 kPa) has been studied and optimum conditions for the preparation of Raney-Ni catalyst for the hydrogenation process have been obtained. The hydrogenation activity of Raney-Ni is found to be strongly influenced by its preparation conditions. Catalytic slurry phase hydrogenation of 0• nitrotoluene over Raney-Ni prepared under nitrophenol using Raney Ni catalyst is an different conditions4 has indicated a strong important commercial process for the production influence of leaching conditions of Ni-Al (50%, AI) of o-aminophenol, an important intermediate for alloy (viz. type of alkali, concentration of alkali, drugs, dyes and pesticides. Nickel aluminium alloy temperature and duration of leaching, and the is commonly used for the preparation of Raney• washing agent (viz. tap water, distilled water, nickel. The alloy contains NiAI3, Ni2A13, and Ni3AI deionised distilled water, 50% ethanol and 95% phases 1.2 and the composition of these phases ethanol) used in the washing of the leached alloy depends on the alloy composition. The non• on the hydrogen content and hydrogenation catalytic part of the alloy (i.e. -

Synthesis of Aldehydes and Ketones by the Dehydrogenation of Alcohols in the Presence of a Raney Nickel/Aluminium Isopropoxide/A
CEJC 2(1)2004 214{219 Communication Synthesisof Aldehydes and Ketones by the Dehydrogenationof Alcohols in the Presence ofa RaneyNickel/ AluminiumIsoprop oxide/Alumina Catalyst 1 1;2 2 1;2 Ilie Ciocan Tarta ,Ioan A.Silberg ,Mircea Vlassa ¤,Ioan Oprean 1 \RalucaRipan "University, Institute ofChemistry, 30F^ ant^aneleStr., RO-3400Cluj-Napoca, Romania 2 \Babes-Bolyai"University, Departmentof Chemistry, 11Arany Janos Str., RO-3400Cluj-Napoca, Romania Received 24June 2003; accepted 13October 2003 Abstract: Thesynthesis ofaldehydes andketones by the dehydrogenationof allylic and secondaryalcohols using aRaney-Ni-Al( i-OPr)3-Al2O3 catalyst without hydrogenacceptors is presented. c Central EuropeanScience Journals. All rights reserved. ® Keywords: dehydrogenation, alcohols, heterogeneous catalysis Raney nickel, aluminium isopropoxide, alumina 1Introduction The oxidation of alcoholsto carbonyl compounds isa fundamental reaction in organic synthesis [1].A number of transition metal-catalyzedprocedures havebeen developed, such as those using ruthenium [2]or palladium[3].A Raney-Nicatalyst isused frequently for the dehydrogenation of secondary alcoholsto ketones because of the high yields. However,this later method has the disadvantage of requiring either high temperatures[4] or largeamounts of hydrogen acceptors ( i:e.cyclohexanone),which are required for completeconversion[5]. Sometimes, noncatalytic quantities of Raney-Nihave been used for the dehydrogenation ofprimary and secondary alcohols[6]. ¤ E-mail:[email protected] I.C.T artaet al. / CentralEuropean Journal of Chemistry 2(1)2004 214{219 215 Our studies show that the dehydrogenation ofthe secondary and allylicalcohols can be achievedwith acatalyticmixture of Raney-Ni:Al( i-OPr)3:Al2O3:substrate (3:5:5:100 weight) without hydrogen acceptors and at temperatures between 120-170 0C (see Table 1). -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Reduction of Ketoximes to Amines by Catalytic Transfer Hydrogenation Using Raney Nickel and 2-Propanol As Hydrogen Donor
University of Tennessee at Chattanooga UTC Scholar Student Research, Creative Works, and Honors Theses Publications 5-2014 Reduction of ketoximes to amines by catalytic transfer hydrogenation using Raney Nickel and 2-propanol as hydrogen donor Katherynne E. Taylor University of Tennessee at Chattanooga Follow this and additional works at: https://scholar.utc.edu/honors-theses Part of the Catalysis and Reaction Engineering Commons, and the Chemistry Commons Recommended Citation Taylor, Katherynne E., "Reduction of ketoximes to amines by catalytic transfer hydrogenation using Raney Nickel and 2-propanol as hydrogen donor" (2014). Honors Theses. This Theses is brought to you for free and open access by the Student Research, Creative Works, and Publications at UTC Scholar. It has been accepted for inclusion in Honors Theses by an authorized administrator of UTC Scholar. For more information, please contact [email protected]. Reduction of Ketoximes to Amines by Catalytic Transfer Hydrogenation Using Raney Nickel® and 2-Propanol! as Hydrogen Donor ! ! By Katherynne E. Taylor ! Departmental Thesis The University of Tennessee at Chattanooga Department of! Chemistry Project Director: Dr. Robert Mebane Examination Date: March 20, 2014 Committee Members: Dr. Jisook Kim Dr. John Lee Dr. Robert Mebane Dr. Abdul! Ofoli ! _________________________________________________________ Project Director _________________________________________________________ Department Examiner _________________________________________________________ Department Examiner _________________________________________________________ -

Electrochemical Hydrogenations in a Solid Polymer Electrolyte Fuel Cell Reactor
Electrochemical Hydrogenations in a Solid Polymer Electrolyte Fuel Cell Reactor Peter N. Pintauro Department of Chemical and Biomolecular Engineering Vanderbilt University Nashville, TN 372235 ARPA-E Workshop: Bridging Renewable Electricity with Transportation Fuels August 27, 2015 Traditional Electrochemical Reductions vs. Electrocatalytic Hydrogenations Traditional Electrochemical Reductions Reactions at a high hydrogen overpotential cathode (e.g., Hg, Zn, Pb, or Cd) to suppress unwanted hydrogen gas evolution. Product selectivity is controlled by the electrode potential - - Org + nH2O + ne → Org-Hn + nOH here the cathode potential is high and we minimize hydrogen evolution - - (nH2O + ne → nHads + nOH ) by the choice of cathode material and by operating at near neutral pH. Electrocatalytic Hydrogenation Reactions at a low hydrogen overpotential catalytic cathode (e.g., Raney nickel, Pt-black, Pd-black). Here we seek to electrochemically generate H species which chemically react with the organic reactant. Selectivity controlled by the chemical catalyst. - - Electrochemical step: nH2O + ne → nHads + nOH Chemical Step: mHads + Org → Org-Hm Unwanted Side Rxn: (n-m)Hads → [(n-m)/2]H2 (gas) Electrochemical Reduction vs. Electrocatalytic Hydrogenation Glucose Sorbitol At a Zn(Hg) cathode, potential = -1.7 to -2.2 V vs SCE, Current Efficiency ~30% At Ra-Ni, potential = -1.1 V vs SCE, Current Efficiency = 60-70% Current Efficiency = (product formed)/(product that should have been made based on the current passed) m F ⋅(moles of Org − H ) Current Efficiency (%) ≡ m ⋅100 curren⋅time Examples of Electrocatalytic Hydrogenation of Aromatic Compounds - using H2O as the Source of H Raney nickel cathode and water/t-butanol solvent; a 1.0 M 6H solution of benzene in 2.0 M tetramethylammonium p- → toluenesulfonate was used (a hydrotropic salt which allows benzene to dissolve in water). -

A Modified Raney Nickel Catalyst and a Process for Preparing Diols by Using the Same
~™ llll III II III I III IIIMII II 1 1 (19) J European Patent Office Office europeen des brevets (11) EP 0 807 464 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) int. CI.6: B01 J 25/00, B01J 25/02, 19.11.1997 Bulletin 1997/47 C07C 29/141 , C07C 29/132 (21) Application number: 96303388.1 (22) Date of filing: 14.05.1996 (84) Designated Contracting States: • LIN, Fwu Shen AT BE CH DE DK ES Fl FR GB GR IE IT LI LU MC Kaohslung (TW) NL PT SE • Chou, Jiunn Yann Kaohslung (TW) (71) Applicant: . Huang, Chian Chong Dairen Chemical Corporation Kaohslung (TW) Taipei (TW) (74) Representative: (72) Inventors: Jones, Helen Marjorie Meredith • Chen, Shien Chang Gill Jennings & Every, Taipei (TW) Broadgate House, • Chu, Cheng Chang 7 Eldon Street Kaohslung (TW) London EC2M 7LH (GB) (54) A modified raney nickel catalyst and a process for preparing diols by using the same (57) The present invention relates to a modified Raney nickel catalyst which can serve as a hydrogena- tion catalyst for hydroxy aldehydes, such as 4-hydroxy- butanal, and 2-methyl-3-hydroxypropanal and hydroxy cyclic ethers such as 2-hydroxy-tetrahydrofuran. Fur- ther, a process for preparing diols by using the modified Raney nickel catalyst is provided. < CO r»- o CO o Q_ LU Printed by Rank Xerox (UK) Business Services 2.14.23/3.4 EP 0 807 464 A1 Description 1 . Field of the Invention 5 The present invention relates to a modified Raney nickel catalyst and a process for preparing diols by using the modified Raney nickel catalyst. -

Investigations with the Use of Raney Nickel Catalyst
INVESTIGATIONS WITH THE USE OF RANEY NICKEL CATALYST I. CHAKGES IN THE HYDROGENATION ACTIVITY OF RANEY KICKEL AS A FUNCTION OF THE TEMPERATURE AND DDRA.TION OF EXTRACTION By. Z. CSUROS, J. PETRO and J. VOROS Department of Organic Chemical Technology, Polytechnic University, Budapest (Received February 15, 1957) Introduction Raney nickel is a much employed hydrogenating catalyst, applied mainly in batch hydrogenations in the liquid phase. Its 'wide-spread use is due to its cheapness and to the relatively simple way of its preparation. Another ad vantage is that Raney nickel is suited for the hydrogenation of many types of compounds. The interest in Raney nickel is attested by the great number of patents and the extensive literature (see later) dealing ,\'ith the preparation of its more active varieties. It is, however, rather difficult to get a dear idea of the ways and means of its preparation failing a reliable basis for comparing the papers and descriptions. Some authors describe the preparation of Raney nickel in divergent ways, ,vithout referring to model compounds. Others demonstrate the hydrogenation activity of catalysts, prepared by them, in the form of results attained ,\'ith model compounds. Only a few research workers publish comparative data ,vith catalysts prepared by other methods. In the above mentioned cases the possibility of compaTison is excluded by the sole fact that almost each reseaTch wOTker uses diffeTent model compounds. In the first part of this paper an attempt is made to study, \\ith othendse identical e:x-perimental factors and model compounds, the changes in the hydro genation activity of Raney nickel as a function of the conditions of preparation, and, respectively, to clear up the order of magnitude of the changes in the activity of the catalyst under altered conditions of preparation (varying the temperature and duration of extraction) and under the action of various additions. -
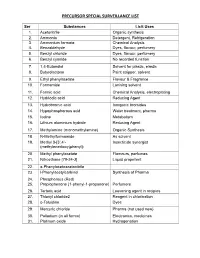
Precursor Special Surveillance List
PRECURSOR SPECIAL1 SURVEILLANCE LIST Ser Substances Licit Uses 1. Acetonitrile Organic synthesis 2. Ammonia Detergent, Refrigeration 3. Ammonium formate Chemical Analysis 4. Benzaldehyde Dyes, flavour, perfumery 5. Benzyl chloride Dyes, flavour, perfumery 6. Benzyl cyanide No recorded function 7. 1,4-Butandiol Solvent for plastic, elastic 8. Butyrolactone Paint stripper, solvent 9. Ethyl phenylacetate Flavour & Fragrance 10. Formamide Lonising solvent 11. Formic acid Chemical Analysis, electroplating 12. Hydriodic acid Reducing Agent 13. Hydrobromic acid Inorganic bromides 14. Hypophosphorous acid Water treatment, pharma 15. Iodine Metabolism 16. Lithium aluminium hydride Reducing Agent 17. Methylamine (monomethylamine) Organic Synthesis 18. N-Methylformamide As solvent 19. Methyl 3-[3’,4’- Insecticide synergist (methylenedioxy)phenyl]- 20. Methyl phenylacetate Flamours, perfumes 21. Nitroethane [79-24-3] Liquid propellent 22. a-Phenylacetoacetonitrile 23. l-Phenylacetylcarbinol Synthesis of Pharma 24. Phosphorous (Red) 25. Propiophenone (1-phenyl-1-propanone) Perfumers 26. Tartaric acid Leavening agent in recipies 27. Thionyl chloride2 Reagent in chlorination 28. o-Toluidine Dyes 29. Mercuric chloride Pharma (not used now) 30. Palladium (in all forms) Electronics, medicines 31. Platinum oxide Hydrogenation 2 32. Raney nickel Reagent 33. Aniline Polyurethane 34. Propionic anhydride Reagent Organic synthesis 35. Propionyl chloride 36. Sodium borohydride Bleaching wood pulp 37. Acetic acid (glacial) Plastic, soap 38. Acetyl chloride Derivatization of organic compounds 39. Ammonium chloride Flavouring agent 40. Calcium carbonate Used medicinally 41. Calcium hydroxide Lime, food prep 42. Calcium chloride Air moisture absorber prep 43. Calcium oxide Construction material etc 44. Manganese dioxide Dry + Cell bty 45. Sodium carbonate Soap, bleaching 45. Sodium hydroxide Salt, soap, Rayon 46. Sodium hypochlorite Bleach, Disinfectant 47. -

Expanding the Scope of Asinger Chemistry Towards Enantiomerically Pure Secondary Amines and Β-Aminothiols Through Chemoenzymatic Derivatization of 3-Thiazolines
Article Expanding the Scope of Asinger Chemistry towards Enantiomerically Pure Secondary Amines and β-Aminothiols through Chemoenzymatic Derivatization of 3-Thiazolines Mentor Hyseni 1, Nadine Zumbrägel 1 , Heribert Offermanns 2,3 and Harald Gröger 1,* 1 Chair of Industrial Organic Chemistry and Biotechnology, Faculty of Chemistry, Bielefeld University, Universitätsstraße 25, 33615 Bielefeld, Germany; [email protected] (M.H.); [email protected] (N.Z.) 2 Grünaustraße 2, 63457 Hanau, Germany; heppoff@gmx.de 3 Former affiliation: Degussa-Hüls AG, Weißfrauenstraße 9, 60287 Frankfurt am Main, Germany * Correspondence: [email protected] Received: 21 August 2019; Accepted: 18 October 2019; Published: 28 October 2019 Abstract: A proof of concept for a novel approach towards enantiomerically highly enriched acyclic secondary amines and β-aminothiols as non-cyclic target molecules when starting from 3-thiazolines as heterocycles is presented. Starting from 2,2,4,5,5-pentamethyl-3-thiazoline, we demonstrated this chemoenzymatic pathway to both of these types of amine molecules, which were isolated as urea derivatives with a non-optimized yield of up to 20%. As a substrate, 2,2,4,5,5-pentamethyl-3-thiazolidine, which was obtained with an enantiomeric excess (ee) of 99% in a biotransformation from the corresponding 3-thiazoline according to a recently developed protocol, was used. For the reductive desulfurization of this substrate leading to a sulfur-free secondary amine, in situ formed Ni2B turned out to be a suitable reducing reagent. However, when using lithium aluminum hydride as a reducing agent, β-aminothiol was obtained. Keywords: amines; β-aminothiols; Asinger chemistry; chemoenzymatic synthesis; heterocycles 1. -

LECTURE 6 Electrophilic Addition to Alkynes Alkynes Are Very Similar To
LECTURE 6 Electrophilic Addition to Alkynes Alkynes are very similar to alkenes in their behaviour towards electrophiles except that they are slightly less reactive despite the lower steric hindrance and the greater number of π-electrons available. Thus addition of HCl or HBr is slow and usually requires catalysis by mercury salts and charcoal: HCl, HgCl2, C HC CH H2C CHCl vinyl chloride The mechanism involves the initial addition of mercuric ion which has a much higher affinity for π-bonds than H+. Subsequent trapping of the vinyl cation introduces the chloride and then the carbon-mercury bond is rapidly cleaved by the proton by an SE2 mechanism: HC C ClHC CH2 Hg2+ -Hg2+ H+ - CH HC CH Cl ClHC + Hg+ Hg Unlike the addition to alkenes, the product still contains a double bond and hence a second addition occurs (generally rapidly since alkenes are more reactive than alkynes): HC CH ClHC CH2 Cl2HC CH3 RADICAL ADDITION TO ALKENES AND ALKYNES The most interesting examples of this reaction occur in the radical- initiated addition of HBr to alkenes, in the polymerisation of alkenes and in the related electron addition to alkynes. (a) Addition of HBr to Alkenes in the Presence of Radical Initiators Radicals derived from the decomposition of radical initiators, such as . peroxides, will abstract hydrogen from HBr to give Br which will add to the alkene to give the most stable radical; a chain is then set up: RO - OR 2RO RO H - Br ROH + Br Br Br R1 R1 H - Br H Br Br 1 Br R1 R The overall result is an anti-Markovnikov addition of HBr i.e.