A Description of Some Oregon Rocks and Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Northwest Newsletter Vol.58 No
TIME SENSITIVE MATERIAL TIME SENSITIVE 84403 S Ogden, UT 4500875S E Burchard,Tom Circulation Societies FederationMineralogical of Northwest Northwest - 2913 Newsletter VOLUME 58, NO. 2 Northwest Federation of Mineralogical Societies FEB 2018 Keith Fackrell President GREETINGS Permit #7Permit McMinnville, McMinnville, OR PAID Postage U.S. Non February? It surely does not appear to be February, but looking at the Calendar it veri- - fies that it really is. Profit Org. I don’t recall any February’s being so dry and so warm for such a long time. It has been cold enough to be a little uncomfortable to do a lot of rock hunting (at least in my local area) so it is a good time to go to your rock pile and pick out some good rocks to cut and polish. It is also the time of year to pre- pare for the upcoming shows. There are many Gem & Mineral Shows on the horizon. Check the listing in your Northwest Federation of Mineralogical Societies (NFMS) Newsletter. If you are traveling beyond the boundaries of NFMS, you can check on the internet for Rock & Gem listing of many shows in any part of the United States. You can enhance your vacation and meet new friends by attending some of the shows wherever you travel. Now, I am asking for your help! I am asking for the NFMS Delegate or the President of your club/Society in the NFMS to make a list consisting of each member in your club/Society who has passed away in the last year since January 1, 2017 to the Present time, along with respective death dates. -

RMSH August 2012 Newsletter.Pdf
VOLUME 47, NO. 8 A UGUST 2012 CHALCEDONY MEETING BY D EAN S AKABE Wednesday This month’s topic is the most worked I call a stone Chalcedony, when it is sort of upon stone in any lapidary operation, translucent and homogeneous in color. August 22 Chalcedony . Chalcedony in a cryptocrys- Such as the Malawi Blue Chalcedony. 6:15-8:00 pm talline form of silica, composed of very Makiki District fine intergrowths of Quartz and Moganite. These are both silica minerals, which differ Park in the respect that quartz has a trigonal Administration crystal structure, while moganite is mono- Building clinic. Chalcedony's standard chemical structure is SiO 2 (Silicon Dioxide). Chalcedony has a waxy luster and is usu- NEXT MONTH ally semitransparent or translucent. It can Wednesday assume a wide range of colors, with the Blue Lace Agate most common seen as white to gray, blue, September 26 or brown ranging from pale to nearly Flourite black. Agates are stones which usually have col- ored layers. These are colored layers of The name "chalcedony" comes from the differently colored layers of Chalcedony. LAPIDARY calcedonius Latin , from a translation from Such as the Blue Lace Agate or Holly Blue Every Thursday khalkedon. the Greek word Unfortu- Agate. 6:30-8:30pm natelly, a connection to the town of Chal- cedon, in Asia Minor could not be found, Forms of Chalcedony are found in all 50 Second-floor Arts but one can always be hopeful. state, occurring in many colors and color and Crafts Bldg combinations. Some of the better known To make things alittle confusing is that ones are: Makiki District Chalcedony and Agate are terms used al- Park most interchangeably, as both are forms of quartz and are both Silicon Dioxide. -

Symposium on Agate and Cryptocrystalline Quartz
Symposium on Agate and Cryptocrystalline Quartz September 10 – 13, 2005 Golden, Colorado Sponsored by Friends of Mineralogy, Colorado Chapter; Colorado School of Mines Geology Museum; and U.S. Geological Survey 2 Cover Photos {top left} Fortification agate, Hinsdale County, Colorado, collection of the Geology Museum, Colorado School of Mines. Coloration of alternating concentric bands is due to infiltration of Fe with groundwater into the porous chalcedony layers, leaving the impermeable chalcedony bands uncolored (white): ground water was introduced via the symmetric fractures, evidenced by darker brown hues along the orthogonal lines. Specimen about 4 inches across; photo Dan Kile. {lower left} Photomicrograph showing, in crossed-polarized light, a rhyolite thunder egg shell (lower left) a fibrous phase of silica, opal-CTLS (appearing as a layer of tan fibers bordering the rhyolite cavity wall), and spherulitic and radiating fibrous forms of chalcedony. Field of view approximately 4.8 mm high; photo Dan Kile. {center right} Photomicrograph of the same field of view, but with a 1 λ (first-order red) waveplate inserted to illustrate the length-fast nature of the chalcedony (yellow-orange) and the length-slow character of the opal CTLS (blue). Field of view about 4.8 mm high; photo Dan Kile. Copyright of articles and photographs is retained by authors and Friends of Mineralogy, Colorado Chapter; reproduction by electronic or other means without permission is prohibited 3 Symposium on Agate and Cryptocrystalline Quartz Program and Abstracts September 10 – 13, 2005 Editors Daniel Kile Thomas Michalski Peter Modreski Held at Green Center, Colorado School of Mines Golden, Colorado Sponsored by Friends of Mineralogy, Colorado Chapter Colorado School of Mines Geology Museum U.S. -

State Rocks Table.Pdf
GATOR GIRL ROCKS THE BEST EVER OFFICIAL STATE ROCK, GEM, MINERAL, FOSSIL, & DINOSAUR SUMMARY TABLE STATE ROCK/(STONE) GEM MINERAL FOSSIL DINOSAUR Basilosaurus cetoides Marble Star Blue Quartz Hematite ALABAMA [whale] 19691 19902 19673 19844 Jade Mammuthus primigenius Gold ALASKA [Nephrite Jade] [woolly mammoth] 1967 1968 1986 STATE ROCKS STATE – Araucarioxylon Turquoise arizonicum ARIZONA 1974 [petrified wood] 1988 Bauxite Diamond Quartz Crystal ARKANSAS 19675 19676 19677 1 Alabama, Act 69-755, Acts of Alabama, September 12, 1969. 2 Alabama, Act 90-203. Acts of Alabama, March 29, 1990. GATORGIRLROCKS.COM GATORGIRLROCKS.COM 3 Alabama, Act 67-503, Acts of Alabama, September 7, 1967. 4 Alabama, Act 84-66, Acts of Alabama, 1984. 5 Arkansas General Assembly 1967. 6 Arkansas General Assembly 1967. 7 Arkansas General Assembly 1967. ©Gator Girl Rocks (2012) – All Rights Reserved GATOR GIRL ROCKS THE BEST EVER OFFICIAL STATE ROCK, GEM, MINERAL, FOSSIL, & DINOSAUR SUMMARY TABLE STATE ROCK/(STONE) GEM MINERAL FOSSIL DINOSAUR Smilodon californicus Serpentine Benitoite Native Gold CALIFORNIA 8 9 10 [saber-tooth cat] 1965 1985 1965 11 1973 Stegosaurus stenops Yule Marble Aquamarine Rhodochrosite COLORADO [dinosaur] 200412 197113 200214 198215 STATE ROCKS STATE Garnet Eubrontes Giganteus – CONNECTICUT [almandine garnet] [dinosaur tracks] 197716 1991 8 California Gov. Code § 425.2. Senate Bill 265 (Laws Chap. 89, Sec. 1) was signed by Govenor Brown on April 20, 1965. California designated the very first official state rock with this legislation (which also created the first official state mineral). 9 California State Legislature October 1, 1985. 10 California Gov. Code § 425.1. Senate Bill 265 (Laws Chap. 89, Sec. -
Alvord Lake Toerswnq Ihitk-Ul,.N Itj:Lnir
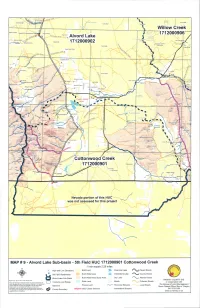
RtflOP' nt T37S Rt3F T37SR36E T37S R3275E 137S R34E TITStSE BloW Pour Willow Creek Babes Canyor Relds BIM Adrnirtistrative SiteFjel NeSs Airstrip / 41 171 2000906 Alvord Lake tOerswnq ihiTk-ul,.n itj:Lnir. Inokoul hluutte\ McDade Ranch 1712000902 I cvwr Twin Hot Spn Os Wilhlants Canyon , ,AEyy.4N seiralNo WARM APRI '738SR37E rIBS R3BE R38E Lower Roux Place err hlomo * / Oaterkirk Rartch - S., Rabbit HOe Mine 7,g.hit 0 Ptace '0 Arstnp 0 0 Ainord Vafley Lavy1ronrb P -o Rriaia .....c I herbbrrr RarrCh C _: 9S R34E Trout Creek CAhn '5- T39S R36E N Oleacireen Place T395 R37F Cruallu Canyon -1 Oleachea Pass T39S R38E \ Stergett Cabot Trout Creek Ranch ty Silvey Adrian Place' Wee Pole Canyon C SPRiNG, WHITEHORSE RANCH LN Pueblo Valley 4040 S Center Ridg Will, reek Pt cLean Cabin orgejadowo Gob 0810 WEIi Reynolds Ranch Owens Randr / I p labor Mocntai /1 °ueblo Mountain A'ir.STWLL Neil Pei S 'i-nWt Mahogany Ri.ge ,haaesot, uSGi, Pee: enn;s *i;i train ' 6868 4 n*a.asay Holloway T4OS R34E T4OS R35E I 1405 6E T405 R37E 'I 14'R38E / i.l:irintoi "Van Hone Basin' Colony Ranch 0 U Lithe Windy Pass z SChrERRYSPR1N. 0 I 1* RO4 - - 'r Denio Basin A' tuitdIhSPIhlNn% ()Conne eme Cottonwood Creek ie;. r' Gller Cabin T4IS R37E L,aticw P ',ik 1712000901 Grassy Basin BLAiRO tih'dlM P4 IS R34E Ago 141S R36E Lung Canyon Ii Middle Canyon Oecio Cemetery East B. ring Corral Canyon ento Nevada portion of this HUC was not assessed for this project MAP # 9 - Alvord Lake Sub-basin - 5th Field HUC 1712000901 Cottonwood Creek 1 inch equals 2.25 miles High and Low Elevations BLM Land Perennial Lake Paved Roads 5th Field Watersheds BLM Wilderness - Intermittent Lake County Roads S Alvord Lake Sub-Basin BLM Wilderness Study Area Dry Lake Arterial Roads HARNEY COUNTY GIS Prnpaeod by: Bryca Mcrnz Dare: Momlu 2006 State Land Marsh "_. -

The Formation of Thundereggs
The Formation of Thundereggs (Lithophysae) The Mechanisms of Rhyolitic Lava Fractionation and Crystallization of Spherulites and the Formation of Star-Shaped Gas Cavities in Lithophysae. Preface Thundereggs were probably first extensively excavated by agate gem enthusiasts and collectors at the original Priday Ranch Deposits, 17 miles north of Madras, Oregon. Indeed, the name “thunderegg” came from the local Warm Springs Indian Tribe who, living on the eastern flanks of the explosive-prone volcanoes such as Mt. Hood, Mt. Jefferson, and Mt. St. Helens, believed that thundereggs were overcast missiles from the thunder-gods in their volcanic quarrels. I can sympathize well with this myth, for I was in Portland, Oregon that Sunday morning of May 18, 1980 when Mt. St. Helens blew with the force of a five-megaton H-Bomb. Fortunately, the ash and tephra drifted eastward on the common west-to-east trade winds, sparing Portland a giant mess of ash to contend with. Since they were first collected, then later excavated at the Priday Ranch (now Richardson’s Rock Ranch, Madras, Oregon), the cutting and lapidary treatment of them has sparked curiosity as to their genesis. While the structure of the whole “eggs” are remarkably similar, their interior agate, opal and quartz cores– along with inclusions of rhyolite shards, moss, and plumes– provide an endless variety, no two being exactly the same. At first, the Indians’ notion of “thunder-god missiles” captured the imagination of early Twentieth Century collectors and a few geologists. At the outcrop of this “original dig,” and at subsequently discovered deposits, the eggs are found in an ashy-like material as solid egg-shaped entities. -

Areas to Explore in the Desert
Areas to Explore in the Desert Clapp Spring Tube Agate Page 2 Ballarat Ghost Town - Death Valley Page 8 Bodie State Historic Park Page 14 N. Black Hill Geode Beds Page 21 Black Agate Thunderegg Mine Page 26 Black Agate Hills Page 32 Calico Ghost Town Page 38 Cinnamon Geode Beds Page 41 Coon Hollow Camping Page 55 Fossil Canyon & Painted Gorge - BLM Page 53 Ghost Town Montgomery City Page 57 Gold Fever in the Desert Anza-Borrego Page 61 Hauser Geode Beds - BLM Page 78 Kyanite Ore Cargo Muchacho Mountains Page 69 McCoy Mountains Limonite Cubes Page 81 Meteorite Treasures Page 86 Lost Breyfogle Mine Page 91 1 Collecting Tube Agate at Clapp Spring Palo Verde Mountains, CA by Delmer G. Ross Clapp Spring is a permanent source of water set in a small oasis of fan palms and mesquite located in some low hills on the northeast flank of the Palo Verde Mountains. It lies approximately nine miles from the community of Palo Verde, California, and perhaps 10 miles west of the Colorado River, the eastern edge of the Colorado Desert. Overlooked on the south by some caves once used by Indians and set at the center of a web of converging animal trails, it is a captivating place. Reflecting the amount of rainfall during the preceding several years, the main pool at the spring varies from a few inches to five feet in diameter and from three to nine inches deep. It is located in the heart of the oasis, at the base of a palm. Except during and a few years after a serious drought, there may be several additional seeps and smaller pools of water in shallow holes dug by wild burros. -

Field Trip Guide to the Middle Eocene Wildcat Mountain Caldera, Ochoco National Forest, Crook County, Oregon by Jason D
Field trip guide to the middle Eocene Wildcat Mountain Caldera, Ochoco National Forest, Crook County, Oregon by Jason D. McClaughry1, Caroline L. Gordon2, and Mark L. Ferns1 1Baker City Field Office, Oregon Department of Geology and Mineral Industries, Baker County Courthouse, 1995 3rd Street, Baker City, Oregon 97814 2Ochoco National Forest, 3160 NE 3rd St., Prineville, Oregon 97754 Overview: This field trip examines the stratigraphy of the middle Eocene Wildcat Mountain caldera exposed east of Prineville, in the Ochoco Mountains of north-central Oregon. Geologic factors that control landslide deposits and mineralization in the east part of the Lower Crooked Basin are discussed. This field trip is 152.7 km (94.9 mi). INTRODUCTION 124o 121o 118o KBML The Wildcat Mountain caldera, in the eastern part of the Lower Portland Crooked Basin, is a volcanic vent complex that collapsed and DCP filled with more than 90 km3 (21.5 mi3) of rhyolitic ash-flow BM 45o WV tuff during the middle Eocene. It is one of only a few caldera 3. Dale Baker City sources for Paleogene ash-flow tuff sheets identified in Oregon and is the first recognized to be Eocene (Figure 1) (Hladky and SFZ 1. 2. CR Unity Wiley, 1993; Hladky, 1996; Ferns and others, 2001; McClaughry Eugene Cougar and Ferns, 2007). Until recently, the lack of Paleogene calderas Prineville Rock WC HC in Oregon was a noteworthy anomaly considering that nu- HLP merous Paleogene calderas have been mapped elsewhere in BFZ OU the western United States. Several Paleogene calderas have 4. BR been identified in adjacent Idaho (McIntyre and others, 1982; KM KBML Leonard and Marvin, 1982; Moye and others, 1988), and many Hart Mountain more have been identified farther south in volcanic fields of the 42o 5. -

Teaching Activities For
Teaching Activities for Questions to Ask Before & after reading the book 2 • Questions to ask before reading the book • What do children already know? With charts • After reading the book – writi ng prompts & thi nki ng it through • Re-read the book looking for more information • Comprehension questions • What do children already know activity conclusion Language Arts 8 • Developing a word wall • Vocabulary game • Putting it all together • Suggested vocabulary list • Silly sentence structure acti vity • Sequenci ng sentence strips • Riddle me this • Word search • Write about it! Science 15 • Rocks and minerals all around us • Understanding the rock cycle • Science journal Math 19 • Mineral math • Sorting and attribute graph Research & Geography 20 • What’s mined i n which state/provi nce • State mineral, rock, & gemstone symbols Other 22 • Julie the Rockhound Bingo Teaching Activities are intended for use at home, in the classroom, and duri ng story-times. Copyright © 2007 by Arbordale Publishing formerly Sylvan Dell Publishing Return to Top Questions to ask children before reading the book • What do you think the book is about by looking at the cover? (or one or two of the inside illustrations) Sometimes it is easy to tell from the cover, other times it is not. • What does the cover illustration show? • Does the title tell you what the book is about? What do children already know? • Young children are naturally inquisitive and are sponges for information. The whole purpose of this activity is to help children verify the information they know (or think they know) and to get them thinking “beyond the box” about a particular subject. -

3. Subbasin Assessment
3. Subbasin Assessment 3.1 Subbasin Overview 3.1.1 General Description Location The John Day Subbasin is located in northeastern Oregon in the southern section of the Columbia Plateau Ecological Province. Its over five million-acre (approximately 5,067,500 acres, or 8000 mi2) drainage area is bound by the Columbia River (Lake Umatilla) to the north, the Blue Mountains to the east, the Aldrich Mountains and Strawberry Range to the south, and the Ochoco Mountains to the west. The John Day Subbasin incorporates portions of Grant, Wheeler, Gilliam, Sherman, Wasco, Jefferson, Umatilla, Morrow, Crook, Harney, Baker and Union counties. The towns within the subbasin with the largest populations include John Day, Prairie City and Condon. Populations of these and the other incorporated towns in the subbasin are listed in Table 2. See Figure 1 for a general overview map of the subbasin. The John Day River flows generally northwest for 284 miles from its origin in the Blue Mountains and joins the Columbia River at river mile (RM) 217 upstream from the town of Rufus. The mainstem portion of the John Day River begins in the Strawberry Mountains in the Malheur National Forest and flows west through the town of John Day (RM 247) and then north from Dayville (RM 212). Major rivers flowing into the mainstem are the North Fork, Middle Fork, and South Fork John Day rivers. The largest tributary to the John Day River is the North Fork, which originates in the Wallowa- Whitman National Forest in the Blue Mountains at elevations near 8000 feet. -
Land and Resource Management Plan Ochoco National Forest and Crooked River National Grassland
United States Department of Agriculture Forest Service Pacific Northwest Region 1989 I Land and Resource Management Plan Ochoco National Forest and Crooked River National Grassland Caring for the Land.. ~~ ~~ ~~ FElS APPENDICES A & I TABLE OF CONTENTS APPENDIX A Issues. Concerns and Opportunities Page Introduction ........................................ A-I Issue Identification ....................................... A-1 Consultation with Others ................................ A4 The Screening Process .............................. A-5 How were the Issues Developed in the Public Involvement Process ............................... A-6 Changes in Issues from Draft to Final ................... A-13 Issues to be Addressed by the EIS ....................... A-I 6 How were the Issues Used in the Development of Alternatives ........... A-24 APPENDIX I Response to Public Comments Page 1 Introduction Summary of Public Participation Activities ....................... 1-1-1 Summary of Comments Received. .................................. 1-14 Issues and Public Comment Summary ........................... 1-1-6 Supplement to the DElS ..................................... 1-1-1 1 Responses to Public Comments ............................ 1-1-12 Changes Made in Response to Public Comments .................... 1-1-13 2 List of Respondents ................................... 1-2-1 3. Summary of Comments and Forest Service Responses Subject Area Index........................................ 13-1 Comments and Responses................................... -

The Geological Newsletter
. :, THE GEOLOGICAL NEWSLETTER G.EOLOGICAL SOCIETY OF THE OREGON· COUNTRY GEOLOGICAL SOCIETY Non-Profit Org. OF THE OREGON COUNTRY U.S. POSTAGE P.O. BOX 907 PAID Portland, Oregon PORTLAND, OR 97207 Permit No. 999 '• GEOLOGICAL SOCIETY OF THE OREGON COUNTRY 1992-1993 ADMINISTRATION ~oaRD OF DIRECTORS President Directors Eveyln Pratt 223-2601 Dr. Donald Botteron (3 years) 245-6251 2971 Canterbury Lane Betty Turner (2 years) 246-3192 Portland, OR 97201 Donald Barr (1 year) 246-2785 President Elect Immediate Past Presidents Esther Kennedy 287-3091 Dr. Walter Sunderland, M.D. 625-6840 6124 NE 28th Ave. DR. Ruth Keen 222-1430 Portland, OR 97211 THE GEOLOGICAL NEWSLETTER Secretary Editor: Donald Barr 246-2785 Shirley O'Dell 245-6339 Calendar: Reba Wilcox 684-7831 3038 SW, Florida Ct. Unit D Business Mgr: Rosemary Kenney 221-0757 Portland, OR 97219 Assist.:M~~~t Steere 246-1670 Treasurer Archie Strong 244-1488 6923 SW 2nd Ave. Portland, OR 97219 ~CTIVITIES CHAIR~ Calligrapher Properties and PA System Clay Kelleher 775-6263 (Lur1cheon) Clay Kelleher 775-6263 Field Trips (Evenings) Booth Joslin 636-2384 Alta B. Fosback 641-6323 Publications Geology Seminars Margaret Steere 246-1670 Richard Bartell 292-6939 Publicity Historian Ruby Turner 234-8730 Charlene Holzwarth 284-3444 Refreshments Hospitality (Friday evenings) (Luncheon) Shirley O'Dell 245-6339 Volunteer (Evening) Shirley O'Dell 245-6339 (Geology Seminar) Library Dorothy Barr 246-2785 Frances Rusche 654-5975 Telephone Connie Newton 255-5225 Past Presidents Panel Volunteer Speakers Bureau Dr. Walter Sunderland, M.D. 625-6840 Bob Richmond 282-3817 Programs Annual Banquet (Luncheon) Clay Kelleher 775-6263 Susan Barrett 639-4583 (Evening) Esther Kennedy 287-3091 Lois Sato 654-7671 ACTIVITIES ANNUAL EVENTS: President's campout-summer.