Structure and Population Dynamics of Myxobolus Infections in Wild and Cultured Oreochromis Niloticus Linnaeus, 1758 in the Noun Division (West-Cameroon)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Limits in the Indigobirds (Ploceidae, Vidua) of West Africa: Mouth Mimicry, Song Mimicry, and Description of New Species
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 162 Species Limits in the Indigobirds (Ploceidae, Vidua) of West Africa: Mouth Mimicry, Song Mimicry, and Description of New Species Robert B. Payne Museum of Zoology The University of Michigan Ann Arbor, Michigan 48109 Ann Arbor MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN May 26, 1982 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN The publications of the Museum of Zoology, University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum techniques, monographic studies, and other contributions not within the scope of the Occasional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan 48109. MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 162 Species Limits in the Indigobirds (Ploceidae, Vidua) of West Africa: Mouth Mimicry, Song Mimicry, and Description of New Species Robert B. -

Dictionnaire Des Villages Du Département Bamoun 42 P
OFFICE DE LA RECHERCHE REfIlUBLIQUE FEDERALE SCIENTIFIQUE ET 'rECHNIQUE DU OUTRE-MER CAMEROUN CENTRE OR5TOM DE YAOUNDE 1 DICTIONNAIRE DES VILLAGES . DU DEPARTEMENT BAMOUN ~prèS la documentation réunie ~ ~ction de Géographiy de l'ORS~ REPERTOIRE GEOGRAPHIQUE DU CAMEROUN FASCICULE n° 16 SH. n° 44 YAOUNDE Janvier 1968 REPERTOIRE GEOGRAPHIQUE DU CAMEROUN Fesc. Tabl.eau de là population du Cameroun, 68 p. Fév. 1965 SH. N° 17 Fasc. 2 Dictionnaire des villages du Dia et Lobo, 89 p. Juin 1965 SH. N° 22 Fasc. 3 Dictionnaire des ~illages de la Haute-Sanaga, 53 p. Août 1965 SH. N° 23 Fasc. 4 Dictionnaire des villages du Nyong et Mfoumou, ~~ p. Octobre 1965 SH. N° ?4 Fasc. 5 Dictionnaire des villages du Nyong et Soo 45 p. Novembre 1965 SH. N° 25 Fasc. 6 Dictionnaire des villages du l'-Jtem 126 p. Décembre 1965 SH. N° 26 Fasc. 7 Dictionnaire des villages de la Mefou 108 p. Janvier 1966 SH. N" 27 Fasc. 8 Dictionnaire des villages du Nyong et Kellé 51 p. Février 1966 5H. N° 28 Fasc. 9 Dictionnaire des villages de la Lékié 71 p. Mars 1966 SH. N° 29 Fasc. 10 Dictionnaire des villages de Kribi P. Mars 1966 SH. N° 30 Fasc. 11 Dictionnaire des villages du Mbam 60 P. Mai 1966 SH. N° 31 Fasc. 12 Dictionnaire des villages de Boumba Ngoko 34 p. Juin 1966 SH 39 Fasc. 13 Dictionnaire des villages de Lom-et-Djérem 35 p. Juillet 1967 SH. 40 Fasc. 14 Dictionnaire des villages de la Kadei 52 p. Août 1967 SH. 41 Fasc. -
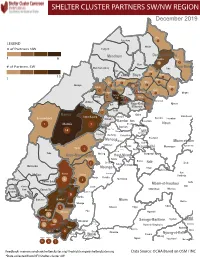
NW SW Presence Map Complete Copy
SHELTER CLUSTER PARTNERS SW/NWMap creation da tREGIONe: 06/12/2018 December 2019 Ako Furu-Awa 1 LEGEND Misaje # of Partners NW Fungom Menchum Donga-Mantung 1 6 Nkambe Nwa 3 1 Bum # of Partners SW Menchum-Valley Ndu Mayo-Banyo Wum Noni 1 Fundong Nkum 15 Boyo 1 1 Njinikom Kumbo Oku 1 Bafut 1 Belo Akwaya 1 3 1 Njikwa Bui Mbven 1 2 Mezam 2 Jakiri Mbengwi Babessi 1 Magba Bamenda Tubah 2 2 Bamenda Ndop Momo 6b 3 4 2 3 Bangourain Widikum Ngie Bamenda Bali 1 Ngo-Ketunjia Njimom Balikumbat Batibo Santa 2 Manyu Galim Upper Bayang Babadjou Malentouen Eyumodjock Wabane Koutaba Foumban Bambo7 tos Kouoptamo 1 Mamfe 7 Lebialem M ouda Noun Batcham Bafoussam Alou Fongo-Tongo 2e 14 Nkong-Ni BafouMssamif 1eir Fontem Dschang Penka-Michel Bamendjou Poumougne Foumbot MenouaFokoué Mbam-et-Kim Baham Djebem Santchou Bandja Batié Massangam Ngambé-Tikar Nguti Koung-Khi 1 Banka Bangou Kekem Toko Kupe-Manenguba Melong Haut-Nkam Bangangté Bafang Bana Bangem Banwa Bazou Baré-Bakem Ndé 1 Bakou Deuk Mundemba Nord-Makombé Moungo Tonga Makénéné Konye Nkongsamba 1er Kon Ndian Tombel Yambetta Manjo Nlonako Isangele 5 1 Nkondjock Dikome Balue Bafia Kumba Mbam-et-Inoubou Kombo Loum Kiiki Kombo Itindi Ekondo Titi Ndikiniméki Nitoukou Abedimo Meme Njombé-Penja 9 Mombo Idabato Bamusso Kumba 1 Nkam Bokito Kumba Mbanga 1 Yabassi Yingui Ndom Mbonge Muyuka Fiko Ngambé 6 Nyanon Lekié West-Coast Sanaga-Maritime Monatélé 5 Fako Dibombari Douala 55 Buea 5e Massock-Songloulou Evodoula Tiko Nguibassal Limbe1 Douala 4e Edéa 2e Okola Limbe 2 6 Douala Dibamba Limbe 3 Douala 6e Wou3rei Pouma Nyong-et-Kellé Douala 6e Dibang Limbe 1 Limbe 2 Limbe 3 Dizangué Ngwei Ngog-Mapubi Matomb Lobo 13 54 1 Feedback: [email protected]/ [email protected] Data Source: OCHA Based on OSM / INC *Data collected from NFI/Shelter cluster 4W. -

Commodification of Care and Its Effects on Maternal Health in the Noun Division (West Region – Cameroon) Ibrahim Bienvenu Mouliom Moungbakou
Moungbakou BMC Medical Ethics 2018, 19(Suppl 1):43 https://doi.org/10.1186/s12910-018-0286-1 RESEARCH Open Access Commodification of care and its effects on maternal health in the Noun division (West Region – Cameroon) Ibrahim Bienvenu Mouliom Moungbakou Abstract Background: Since the mid-1980s, there has been a gradual ethical drift in the provision of maternal care in African health facilities in general, and in Cameroon in particular, despite government efforts. In fact, in Cameroon, an increasing number of caregivers are reportedly not providing compassionate care in maternity services. Consequently, many women, particularly the financially vulnerable, experience numerous difficulties in accessing these health services. In this article, we highlight the unequal access to care in public maternity services in Cameroon in general and the Noun Division in particular. Methods: For this study, in addition to documentary review, two qualitative data collection techniques were used: direct observation and individual interviews. Following the field work, the observation data were categorized and analyzed to assess their relevance and significance in relation to the topics listed in the observation checklist. Interviews were recorded using a dictaphone; they were subsequently transcribed and the data categorized and coded. After this stage, an analysis grid was constructed for content analysis of the transcripts, to study the frequency of topics addressed during the interviews, as well as divergences and convergences among the respondents. Results: The results of this data analysis showed that money has become the driving force in service provision. As such, it is the patient’s economic capital that counts. Considered “clients”, pregnant women without sufficient financial resources wait long hours in corridors; some die in pain under the indifferent gaze of the professionals who are supposed to take care of them. -

Effect of Season on Myxosporean Infections
e Rese tur arc ul h c & a u D q e A v Fonkwa et al. J Aquac Res Development 2018, 9:5 e f l o o l p Journal of Aquaculture a m DOI: 10.4172/2155-9546.1000533 n r e u n o t J Research & Development ISSN: 2155-9546 Research Article Article Open Access Effect of Season on Myxosporean Infections in Oreochromis niloticus Linnaeus, 1758 (Cichlidae) at MAPE Dam in Adamawa, Cameroon Fonkwa Georges1,3*, Lekeufack Folefack Guy Benoît2, Tchuinkam Timoléon3, Ishtiyaq Ahmad4 and Tchoumboue Joseph1 1Applied Hydrobiology and Ichthyology Research Unit, Department of Animal production, Faculty of Agronomy and Agricultural Science, University of Dschang, P.O. Box 222, Dschang-Cameroon 2Laboratory of General Biology, Faculty of Science, University of Yaoundé I, P. O. Box 812, Yaoundé-Cameroon 3Vector Borne Diseases Laboratory of the Applied Biology and Ecology Research Unit, Department of Animal Biology, Faculty of Science, University of Dschang P.O. Box 67, Dschang-Cameroon 4DST Sponsored Fish Nutrition Laboratory, Department of Zoology, University of Kashmir, Sringagr, J&K, India-190006 Abstract In order to contribute to a better understanding of the effect of season on Myxosporean infections so as to elaborate prevention and control strategies, 350 Oreochromis niloticus specimens were sampled from May 2016 to May 2017 from the MAPE dam (Adamawa-Cameroon) and the prevalence of infection was determined after classical examination of fish. A total of 12 species of Myxosporeans belonging to the genus Myxobolus were identified. Irrespective of the parasite species, the prevalence was significantly higher in the dry season (52.94%) than the rainy season (39.59%). -

Wetland Hydrodynamics Using Interferometric Synthetic Aperture Radar, Remote Sensing, and Modeling
WETLAND HYDRODYNAMICS USING INTERFEROMETRIC SYNTHETIC APERTURE RADAR, REMOTE SENSING, AND MODELING DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Hahn Chul Jung, M. S. Graduate Program in Geological Sciences The Ohio State University 2011 Dissertation Committee: Dr. Douglas Alsdorf, Advisor Dr. Ralph R.B. von Frese Dr. Kenneth C. Jezek Dr. C.K. Shum Copyright by Hahn Chul Jung 2011 ABSTRACT The wetlands of low-land rivers and lakes are massive in size and in volumetric fluxes, which greatly limits a thorough understanding of their flow dynamics. The complexity of floodwater flows has not been well captured because flood waters move laterally across wetlands and this movement is not bounded like that of typical channel flow. The importance of these issues is exemplified by wetland loss in the Lake Chad Basin, which has been accelerated due primarily to natural and anthropogenic processes. This loss makes an impact on the magnitude of flooding in the basin and threatens the ecosystems. In my research, I study three wetlands: the Amazon, Congo, and Logone wetlands. The three wetlands are different in size and location, but all are associated with rivers. These are representative of riparian tropical, swamp tropical and inland Saharan wetlands, respectively. First, interferometric coherence variations in JERS-1 (Japanese Earth Resources Satellite) L-band SAR (Synthetic Aperture Radar) data are analyzed at three central Amazon sites. Lake Balbina consists mostly of upland forests and inundated trunks of dead, leafless trees as opposed to Cabaliana and Solimões-Purús which are dominated by flooded forests. -

Community Media Is 10% Media and 90% Community a Mapping Project on the Potentials of Community Media in Cameroon in Times of Conflict
1 CCMN Cameroon Community Media Network Community Media is 10% Media and 90% Community A Mapping Project on the Potentials of Community Media in Cameroon in Times of Conflict A project by: Financed by: P.2 Editorial P.5 Community Media as Conveyer of a peaceful solution to the current Anglophone Crisis P.8 Community Media produce Open Spaces for Communities in Times of War P.12 CCMN In numbers P.15 List of CCMN Members P.17 Who is CCMN P.19 We’ve come a long way! We Need Women’s voices in media P.22 CCMN Staff P.24 CCMN and Peace Journalism Peace Journalistic Approaches and Professionalism can lower P.26 Risk for Journalists in Conflict Situation P.28 CCMN in South West/Littoral P.30 CCMN in North West/West P.34 Program Content P.36 Languages in the Network 2 3 There is no better time than now to sound the Community media play a crucial role in favouring clarion call for peace public debate and preserving the country’s precious diversity of people and cultures RT REV FONKI SAMUEL JOHANNES SINGER Moderator of the PCC Project Officer Cameroon Bread for the World Over the past years and decades, media consumption At Bread for the World, we are convinced that Cameroon Community Media “ been out of school for the past three academic years – has changed profoundly all over the globe. We have Cameroon’s community media precisely play a crucial Network (CCMN) which has brought the call for peace is urgent! access to an ever broadening range of international role in favouring public debate and preserving the together media practitioners and We are grateful to our partners, Bread for the World media and often receive the same „latest news“ – country’s precious diversity of people and cultures. -

Lake Chad Basin
Integrated and Sustainable Management of Shared Aquifer Systems and Basins of the Sahel Region RAF/7/011 LAKE CHAD BASIN 2017 INTEGRATED AND SUSTAINABLE MANAGEMENT OF SHARED AQUIFER SYSTEMS AND BASINS OF THE SAHEL REGION EDITORIAL NOTE This is not an official publication of the International Atomic Energy Agency (IAEA). The content has not undergone an official review by the IAEA. The views expressed do not necessarily reflect those of the IAEA or its Member States. The use of particular designations of countries or territories does not imply any judgement by the IAEA as to the legal status of such countries or territories, or their authorities and institutions, or of the delimitation of their boundaries. The mention of names of specific companies or products (whether or not indicated as registered) does not imply any intention to infringe proprietary rights, nor should it be construed as an endorsement or recommendation on the part of the IAEA. INTEGRATED AND SUSTAINABLE MANAGEMENT OF SHARED AQUIFER SYSTEMS AND BASINS OF THE SAHEL REGION REPORT OF THE IAEA-SUPPORTED REGIONAL TECHNICAL COOPERATION PROJECT RAF/7/011 LAKE CHAD BASIN COUNTERPARTS: Mr Annadif Mahamat Ali ABDELKARIM (Chad) Mr Mahamat Salah HACHIM (Chad) Ms Beatrice KETCHEMEN TANDIA (Cameroon) Mr Wilson Yetoh FANTONG (Cameroon) Mr Sanoussi RABE (Niger) Mr Ismaghil BOBADJI (Niger) Mr Christopher Madubuko MADUABUCHI (Nigeria) Mr Albert Adedeji ADEGBOYEGA (Nigeria) Mr Eric FOTO (Central African Republic) Mr Backo SALE (Central African Republic) EXPERT: Mr Frédèric HUNEAU (France) Reproduced by the IAEA Vienna, Austria, 2017 INTEGRATED AND SUSTAINABLE MANAGEMENT OF SHARED AQUIFER SYSTEMS AND BASINS OF THE SAHEL REGION INTEGRATED AND SUSTAINABLE MANAGEMENT OF SHARED AQUIFER SYSTEMS AND BASINS OF THE SAHEL REGION Table of Contents 1. -

Pdf | 300.72 Kb
Report Multi-Sector Rapid Assessment in the West and Littoral Regions Format Cameroon, 25-29 September 2018 1. GENERAL OVERVIEW a) Background What? The humanitarian crisis affecting the North-West and the South-West Regions has a growing impact in the bordering regions of West and Littoral. Since April 2018, there has been a proliferation of non-state armed groups (NSAG) and intensification of confrontations between NSAG and the state armed forces. As of 1st October, an estimated 350,000 people are displaced 246,000 in the South-West and 104,000 in the North-West; with a potential increment due to escalation in hostilities. Why? An increasing number of families are leaving these regions to take refuge in Littoral and the West Regions following disruption of livelihoods and agricultural activities. Children are particularly affected due to destruction or closure of schools and the “No School” policy ordered by NSAG since 2016. The situation has considerably evolved in the past three months because of: i) the anticipated security flashpoints (the start of the school year, the “October 1st anniversary” and the elections); ii) the increasing restriction of movement (curfew extended in the North-West, “No Movement Policy” issued by non-state actors; and iii) increase in both official and informal checkpoints. Consequently, there has been a major increase in the number of people leaving the two regions to seek safety and/or to access economic and educational opportunities. Preliminary findings indicate that IDPs are facing similar difficulties and humanitarian needs than the one reported in the North-West and the South-West regions following the multisectoral needs assessment done in March 2018. -

Proceedingsnord of the GENERAL CONFERENCE of LOCAL COUNCILS
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN Peace - Work - Fatherland Paix - Travail - Patrie ------------------------- ------------------------- MINISTRY OF DECENTRALIZATION MINISTERE DE LA DECENTRALISATION AND LOCAL DEVELOPMENT ET DU DEVELOPPEMENT LOCAL Extrême PROCEEDINGSNord OF THE GENERAL CONFERENCE OF LOCAL COUNCILS Nord Theme: Deepening Decentralization: A New Face for Local Councils in Cameroon Adamaoua Nord-Ouest Yaounde Conference Centre, 6 and 7 February 2019 Sud- Ouest Ouest Centre Littoral Est Sud Published in July 2019 For any information on the General Conference on Local Councils - 2019 edition - or to obtain copies of this publication, please contact: Ministry of Decentralization and Local Development (MINDDEVEL) Website: www.minddevel.gov.cm Facebook: Ministère-de-la-Décentralisation-et-du-Développement-Local Twitter: @minddevelcamer.1 Reviewed by: MINDDEVEL/PRADEC-GIZ These proceedings have been published with the assistance of the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für internationale Zusammenarbeit (GIZ) GmbH in the framework of the Support programme for municipal development (PROMUD). GIZ does not necessarily share the opinions expressed in this publication. The Ministry of Decentralisation and Local Development (MINDDEVEL) is fully responsible for this content. Contents Contents Foreword ..............................................................................................................................................................................5 -

Notes on the Political Sociology of Chad
The Dynamics of National Integration: Ladiba Gondeu Working Paper No. 006 (English Version) THE DYNAMICS OF NATIONAL INTEGRATION: MOVING BEYOND ETHNIC CONFLICT IN A STATE-IN-WAITING LADIBA GONDEU October 2013 The Sahel Research Group, of the University of Florida’s Center for African Studies, is a collaborative effort to understand the political, social, economic, and cultural dynamics of the countries which comprise the West African Sahel. It focuses primarily on the six Francophone countries of the region—Senegal, Mauritania, Mali, Burkina Faso, Niger, and Chad—but also on in developments in neighboring countries, to the north and south, whose dy- namics frequently intersect with those of the Sahel. The Sahel Research Group brings together faculty and gradu- ate students from various disciplines at the University of Florida, in collaboration with colleagues from the region. Acknowledgements: This work is the fruit of a four month academic stay at the University of Florida Center for African Studies as a Visiting Scholar thanks to the kind invitation of the Profesor Leonardo A. Villalón, Coordinator of the Sahel Research Group. I would like to express my deep appreciation and gratitude to him and to his team. The ideas put forth in this document are mine and I take full responsibility for them. About the Author: Ladiba Gondeu, Faculty Member in the Department of Anthropology at the University of N’Djamena, and Doctoral Candidate, Paris School of Graduate Studies in Social Science for Social Anthropology and Ethnology. Ladiba Gondeu is a Chadian social anthropologist specializing in civil society, religious dynamics, and project planning and analysis. -

Draft Framework Guidelines
CAMEROON NATIONAL OIL SPILL CONTINGENCY PLAN September 2004 CAMEROON NATIONAL OIL SPILL CONTINGENCY PLAN This plan is brought into effect by the powers assigned to me in terms of the Decree of Application promulgated under the Environmental Framework Law (Law No. 96/12 of 5 August 1996) Signed: ............................................................. Date:.......................................... His Excellency:................................................. CSIR Report No. ENV-S-C2004-072 PREPARED BY: PREPARED FOR: CSIR Environmentek Comité de Pilotage et de Suivi des P O Box 320 Stellenbosch Pipelines (CPSP) South Africa 7599 B.P. 955 Yaoundé CONTACT PERSON: Cameroon Mike Burns Tel: 27 21-888 2404 Fax: 27 21-888 2693 Email: [email protected] September 2004 Revision: 0 Cameroon National Oil Spill Contingency Plan FOREWORD The National Oil Spill Contingency Plan (NOSCP) for Cameroon has been prepared in response to an historic situation that has prevailed within the country, in which a variety of up- and downstream activities associated with the hydrocarbon sector, as well as other activities – for example, shipping - have been conducted in the absence of best-practice strategies and clearly defined roles, responsibilities and communication structures designed to ensure an effective response to oil spills.1 The Chad-Cameroon pipeline project is an important catalyst that has triggered the need for the NOSCP. This project, which has been initiated to develop a 1 billion barrel hydrocarbon reserve within Chad, will result in the conveyance by pipeline of some 225 000 barrels per day of heavy crude oil through Cameroonian territory and its discharge at a marine terminal located within Cameroon’s coastal waters. Clearly, the issue of risk associated with potential pipeline product spills arises and, therefore, justifies the need for the NOSCP.