175 Post-Copulatory Behaviour in Calopteryx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Impact of Environmental Changes on the Behavioral Diversity of the Odonata (Insecta) in the Amazon Bethânia O
www.nature.com/scientificreports OPEN Impact of environmental changes on the behavioral diversity of the Odonata (Insecta) in the Amazon Bethânia O. de Resende1,2*, Victor Rennan S. Ferreira1,2, Leandro S. Brasil1, Lenize B. Calvão2,7, Thiago P. Mendes1,6, Fernando G. de Carvalho1,2, Cristian C. Mendoza‑Penagos1, Rafael C. Bastos1,2, Joás S. Brito1,2, José Max B. Oliveira‑Junior2,3, Karina Dias‑Silva2, Ana Luiza‑Andrade1, Rhainer Guillermo4, Adolfo Cordero‑Rivera5 & Leandro Juen1,2 The odonates are insects that have a wide range of reproductive, ritualized territorial, and aggressive behaviors. Changes in behavior are the frst response of most odonate species to environmental alterations. In this context, the primary objective of the present study was to assess the efects of environmental alterations resulting from shifts in land use on diferent aspects of the behavioral diversity of adult odonates. Fieldwork was conducted at 92 low‑order streams in two diferent regions of the Brazilian Amazon. To address our main objective, we measured 29 abiotic variables at each stream, together with fve morphological and fve behavioral traits of the resident odonates. The results indicate a loss of behaviors at sites impacted by anthropogenic changes, as well as variation in some morphological/behavioral traits under specifc environmental conditions. We highlight the importance of considering behavioral traits in the development of conservation strategies, given that species with a unique behavioral repertoire may sufer specifc types of extinction pressure. Te enormous variety of behavior exhibited by most animals has inspired human thought, arts, and Science for centuries, from rupestrian paintings to the Greek philosophers. -

From the Ebony Jewelwing Damsel
Comp. Parasitol. 71(2), 2004, pp. 141–153 Calyxocephalus karyopera g. nov., sp. nov. (Eugregarinorida: Actinocephalidae: Actinocephalinae) from the Ebony Jewelwing Damselfly Calopteryx maculata (Zygoptera: Calopterygidae) in Southeast Nebraska, U.S.A.: Implications for Mechanical Prey–Vector Stabilization of Exogenous Gregarine Development RICHARD E. CLOPTON Department of Natural Science, Peru State College, Peru, Nebraska 68421, U.S.A. (e-mail: [email protected]) ABSTRACT: Calyxocephalus karyopera g. nov., sp. nov. (Apicomplexa: Eugregarinorida: Actinocephalidae: Actino- cephalinae) is described from the Ebony Jewelwing Damselfly Calopteryx maculata (Odonata: Zygoptera: Calopteryigidae) collected along Turkey Creek in Johnson County, Nebraska, U.S.A. Calyxocephalus gen. n. is distinguished by the form of the epimerite complex: a terminal thick disk or linearly crateriform sucker with a distal apopetalus calyx of petaloid lobes and a short intercalating diamerite (less than half of the total holdfast length). The epimerite complex is conspicuous until association and syzygy. Association occurs immediately before syzygy and is cephalolateral and biassociative. Gametocysts are spherical with a conspicuous hyaline coat. Lacking conspicuous sporoducts they dehisce by simple rupture. Oocysts are axially symmetric, hexagonal dipyramidic in shape with slight polar truncations, bearing 6 equatorial spines, 1 at each equatorial vertex and 6 terminal spines obliquely inserted at each pole, 1 at each vertex created by polar truncation. The ecology of the C. karyopera–C. maculata host–parasite system provides a mechanism for mechanical prey–vector stabilization of exogenous gregarine development and isolation. KEY WORDS: Odonata, Zygoptera, Calopteryx maculata, damselfly, Apicomplexa, Eugregarinida, Actinocephalidae, Actinocephalinae, Calyxocephalus karyopera, new genus, new species, parasite evolution, biodiversity, species isolation, vector, transmission. -
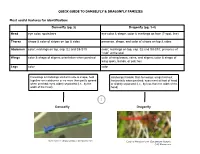
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Calopteryx Splendens, C. Virgo) During Social Interaction: a Signal Or a Symptom?
Russian Entomol. J. 25(1): 103–120 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2016 Behaviour of two demoiselle species (Calopteryx splendens, C. virgo) during social interaction: a signal or a symptom? Ïîâåäåíèå äâóõ âèäîâ ðàâíîêðûëûõ ñòðåêîç êðàñîòêè áëåñòÿùåé Calopteryx splendens Harris, 1780 è êðàñîòêè-äåâóøêè C. virgo Linnaeus, 1758 (Odonata, Calopterygidae) E.N. Panov1*, A.S. Opaev1, E.Yu. Pavlova1, V.A. Nepomnyashchikh2 Å.Í. Ïàíîâ1*, À.Ñ. Îïàåâ1, Å.Þ. Ïàâëîâà1, Â.À. Íåïîìíÿùèõ2 1 A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Leninsky prosp., 33, Moscow, 119071 Russia. 1 Институт проблем экологии и эволюции им. А.Н. Северцова РАН, Ленинский просп., 33, Москва 119071 Россия. 2 Institute of Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742 Russia. 2 Институт биологии внутренних вод РАН, п. Борок, Ярославская область, 152742 Россия. * Corresponding author. E-mail: [email protected] KEY WORDS: damselfly, agonistic behaviour, evolution of courtship behaviour, sexual selection. КЛЮЧЕВЫЕ СЛОВА: стрекоза, агонистическое поведение, эволюция поведения ухаживания, половой отбор. ABSTRACT. Based on the analysis of video record- ного характера. Подвержены серьезной критике ings, obtained during five field seasons, we suggest a трактовки функциональных объяснений поведения pattern of analytical description of damselfly (Zygoptera: стрекоз, господствующие в современной литерату- Calopterygidae) behaviour in a broad spectrum of so- ре по этим насекомым и основанные на откровен- cial contexts. The arrays of motor coordinations and ном антропоморфизме. Этому подходу противопо- their place in social interactions are studied compara- ставлен совершенно иной, призывающий к возвра- tively and in the temporal dynamic. The first description ту к оставленным в последние десятилетия методам of the full set of behaviours of Banded Demoiselles и принципам, разработанным ранее в рамках поле- Calopteryx splendens is given; the respective data for вой и аналитической этологии. -

Ebony Jewelwing, Black-Winged Damselfly
EENY-693 Ebony Jewelwing, Black-Winged Damselfly (suggested common names) Calopteryx maculata (Beauvois, 1807) (Insecta: Odonata: Calopterygidae)1 Alfred Runkel, Nathan Burkett-Cadena, and Andrea Lucky2 Introduction Calopteryx maculata (Beauvois), the ebony jewelwing, is a large damselfly in the family Calopterygidae that is endemic to eastern North America. The ebony jewelwing has an iridescent green body with dark wings (Figures 1–3). Wings of the male ebony jewelwing are completely black, while wings of the female are smoky bronze with a distinct white spot (pterostigma) at the outer edge of the forewing (Figure 4). The combination of iridescent green body and dark wings distinguish this species from all other damselflies in the family Calopterygidae, and from other damselflies in North America. The ebony jewelwing is not listed as a species of concern. Figure 2. Male ebony jewelwing, Calopteryx maculata (Beauvois), resting on a leaf (lateral view). Credits: Nathan Burkett-Cadena, UF/IFAS Florida Medical Entomology Laboratory Synonymy Agrion maculatum (Beauvois 1805) Agrion virginica (Westwood 1837) Calopteryx virginica (Westwood 1837) Figure 1. Male ebony jewelwing, Calopteryx maculata (Beauvois), Calopteryx holosericea (Burmeister 1839) resting on a leaf (lateral view). Credits: Alfred Runkel, UF/IFAS Florida Medical Entomology Laboratory Calopteryx opaca (Say 1839) 1. This document is EENY-693, one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. Original publication date November 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. This document is also available on the Featured Creatures website at http:// entnemdept.ifas.ufl.edu/creatures/. 2. Alfred Runkel, field assistant, Department of Entomology and Nematology, Florida Medical Entomology Laboratory; Nathan Burkett-Cadena, Department of Entomology and Nematology, Florida Medical Entomology Laboratory; and Andrea Lucky, Department of Entomology and Nematology; UF/IFAS Extension, Gainesville, FL 32611. -

Preliminary Survey and Inventory of Odonates of the Primitive Texas Big Thicket-With Notes on Their Distribution During the 2010-2011 Texas Drought
Survey of Big Thicket Odonates: Preliminary Survey and Inventory of Odonates of the Primitive Texas Big Thicket-with notes on their distribution during the 2010-2011 Texas Drought *Autumn J. Smith-Herron and **Tamara J. Cook *Texas Research Institute for Environmental Studies; **Department of Biological Sciences Sam Houston State University, Huntsville, Texas 77341-2506 *[email protected] Abstract. — As part of an ongoing survey of odonate diversity in the Big Thicket and Primitive Big Thicket regions of east-central Texas, U.S.A. (including some collections in west and south Texas), we made some interesting observations about odonate populations during the drought year 2010-2011in comparison to previous (non-drought) years. Our current research not only documents odonate diversity, but the gregarine parasite communities within (parasite communities nested within odonate communities). In part, this has allowed us to document trends in odonate species success over several years of field collections as well as discover/describe new parasite species. The most interesting trend however is noted during the 2010-2011 drought across Texas. In addition, during these surveys, we have discovered and documented several new odonate county records as well as one state record. Introduction. — During a year-long survey and inventory of Odonata from the Big Thicket National Preserve and surrounding areas of southeast Texas, we noted a significant decline in diversity within certain groups of Zygoptera in comparison to previous years. The onset of the 2010-2011 collecting season was initiated as a result of funding provided through the Big Thicket Association, and served to document the odonate fauna from the Big Thicket National Preserve (and Primitive Big Thicket areas). -

A Preliminary Checklist of the Damselflies of Virginia, with Notes on Distribution and Seasonality (Odonata: Zygoptera)
Banisteria, Number 4, 1994 © 1994 by the Virginia Natural History Society A Preliminary Checklist of the Damselflies of Virginia, with Notes on Distribution and Seasonality (Odonata: Zygoptera) Steven M. Roble Division of Natural Heritage Virginia Department of Conservation and Recreation 1500. E. Main Street, Suite 312 Richmond, VA 23219 Virginia has a diverse fauna of aquatic insects, ginia's boundaries occurred in 1862 (R. L. Hoffman, pers. although much additional inventory is needed to fully comm.), subsequent authors (e.g., Muttkowski, 1910; catalog this diversity. Species new to science continue to Needham & Heywood, 1929) failed to account for it in be discovered in the state (e.g., Kondratieff & Kirchner, their range descriptions for several species. Valid Virginia 1994). The aquatic groups treated in the "Insects of records have since been published for all but one (Isch- Virginia" series to date are limited to the true bugs and nura prognata) of these species. several families of beetles and flies (Bobb, 1974; Gladney The following annotated checklist of the state's & Turner, 1969; Matta, 1974, 1976; Michael & Matta, damselfly fauna should be considered as preliminary. I 1977; Pechuman, 1973). Species checklists have been have not conducted an exhaustive search of available compiled for the stoneflies (Kondratieff & Voshell, 1979; collections in preparing this list. In addition to published Kondratieff & Kirchner, 1987), mayflies (Kondratieff & records, my sources are primarily limited to the collection Voshell, 1983), caddisflies (Parker & Voshell, 1981), and of the United States National Museum of Natural dragonflies (Carle, 1978, 1979, 1982) of the state. The History, Washington, D.C. (abbreviated as USNM present contribution is the first attempt to publish a hereafter) and specimens collected statewide from 1988- comprehensive list of the damselfly species known from 1994 by the zoological staff of the Division of Natural Virginia. -

The Woolston Eyes Conservation Group
The Woolston Eyes Conservation Group Annual Report 2014 WOOLSTON EYES CONSERVATION GROUP ANNUAL REPORT 2014 CONTENTS Page Chairman’s Report B. Ankers 2 Weather B. Martin 3 Systematic List B. Martin 6 D. Hackett D. Spencer D. Riley D. Bowman WeBS Counts B. Martin 54 Ringing Report M. Miles 56 Recoveries and Controls M. Miles 63 Migration Watch D. Steel 71 Butterfly Report D. Hackett 75 Odonata Report Brian Baird 82 Willow Tit Report Alan Rustell 90 Heteroptera and Diptera Phil Brighton 92 Mammals D. Bowman 99 Acknowledgments D. Bowman 104 Officers of Woolston Eyes Conservation Group 105 © No part of this report may be reproduced in any way without the prior permission of the Editor. 1 CHAIRMAN’S REPORT A special welcome to all those members taking the Annual Report for the first time now that it is available online. I am sure you will find it an enjoyable and informative read. Our income comes from the sale of permits and any grants that we manage to acquire, and all monies are put back into the Reserve. As you are aware, the management of the Reserve has always been undertaken by volunteers, but specialist work is done by our contractors so this is where a lot of our income is spent. I would therefore like to thank all of you for your continued support in renewing your permits, sending in donations and, in two cases, for bequests leaving money to the Reserve in wills. You will see on the cover of our report the wonderful drawing produced free of charge to us by the highly thought of wildlife artist Colin Woolf. -

Download Vol. 16, No. 2
r.'. , - ''.7--, 9 . -1 1.11 1 of the FLORIDA STATE MUSEUM Biological Sciences Volume 16 1972 Number 2 THE DAMSELFLIES (Zygoptera) of TEXAS Clifford Johnson -1 - I I 1. UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF THE FLORIDA STATE MUSEUM, BIOLOGICAL SCIENCES, are published at irregular intervals. Volumes contain about 300 pages and are not necessarily completed in any one calendar year. OLIVER L. AUSTIN , JR ., Editor FRED G. THOMPSON, Managing Editor Consultants for this issue: HARRY K. CLENCH DENNIS R. PAULSON Communications concerning purchase or exchange of the publication and all manuscripts should be addressed to the Managing Editor of the Bulletin, Florida State Museum, Museum Road, University of Florida, Gainesville, Florida 32601. Publication date: 18 January, 1972 Price: $1.25 THE DAMSELFLIES (ZYGOPTERA) oF TEXAS CLIFFORD JOHNSON , SYNOPSIS: This report presents an identification guide to adult damselflies oc- curring in Texas. Illustrated characters, a guide to morphological terminology, and short text support the diagnostic keys. The text gives geographical range and habitat preferences for each group. Distribution data appear by county for each species and reveal patterns of convergence between east and west faunas. TABLE OF CONTENTS 56 INTRODUCTION ACKNOWLEDGEMENTS 57 METHODS . 57 KEY TO THE FAMILIES 62 LESTIDAE.. 6 Archilestes 63 Lestes 65 CALOPTERYCIDAE 69 Calopteryx 69 71 Hetaerina PROTONEURIDAE 73 COENAGRIONIDAE 74 Argia 78 Enallagma 93 Ischnum 1 ()0 Smaller Genera 107 Di SCUSSION 111 LITERATURE CITED ... 115 APPENDIX 117 The author is Associate Professor in the Department of Zoology, University of Florida, Gainesville, Florida, 32601. Manuscript accepted 22 March 1971 - Ed. Johnson, Clifford. -

Download Vol. 15, No. 2
BULLETIN OF THE FLORIDA STATE MUSEUM BIOLOGICAL SCIENCES Volume 15 Number 2 DIAGNOSTIC KEYS AND NOTES ON THE DAMSELFLIES (ZYGOPTERA) OF FLORIDA Clifford Johnson and Mintef J. Westfall, Jr. \/821/ UNIVERSITY OF FLORIDA Gainesville 1970 Numbers of the BULLETIN OF THE FLORIDA STATE MUSEUM are pub- lished at irregular intervals. Volumes contain about 300 pages and are not neces- sarily completed in any one calendar year. WALTER AUFFENBERG, Managing Editor OLIvER L. AusTIN, JR., Editor Consultants for this issue: LEWIS BERNER HARRY K. CLENCH Communications concerning purchase or exchange of the publication and all manuscripts should be addressed to the Managing Editor of the Bulletin, Florida State Museum, Seagle Building, Gainesville, Florida 32601. Published 10 April 1970 Price for this issue $.80 DIAGNOSTIC KEYS AND NOTES ON THE DAMSELFLIES ( ZYGOPTERA) OF FLORIDA CLIFFORD JOHNSON AND MINTER J. WESTFALL, JR.1 SYNOPSIS: This study presents a current species list and identification guide to the -45 species of damselflies (.Zygoptera) occurring in Florida, a guide to mor- phological terms, and a short text improving accuracy of determinations. Illus- trated characters of each species and. sex accompany the keys. Color patterns, behavior traits, and habitat preferences serving as identifying characters in the field support the keys. The text provides general distribution within the state for eacb species, and references to larval descriptions. Attention is directed to problems in damselfly ecology. TABLE. OF CONTENTS INTRODUCTION' 45 . COENAGRIONIDAE 59 ACKNOWLEDGMENTS - - 46 ATgia - 63 METHODS AND MATERIALS 46 Enallagma 70 KEY To THE FAMILIES . _ 51 Ischnura 79 LESTIDAE, Lestes 51 SMALLER GENERA 83 CALOPTERYGIDAE 54 85 Calopteryx 56 DIscussION Hetaerina 57 LITERATURE CITED 87 INTRODUCTION This report presents a current list and identification guide to adult damselHies in Florida. -

IDF-Report 92 (2016)
IDF International Dragonfly Fund - Report Journal of the International Dragonfly Fund 1-132 Matti Hämäläinen Catalogue of individuals commemorated in the scientific names of extant dragonflies, including lists of all available eponymous species- group and genus-group names – Revised edition Published 09.02.2016 92 ISSN 1435-3393 The International Dragonfly Fund (IDF) is a scientific society founded in 1996 for the impro- vement of odonatological knowledge and the protection of species. Internet: http://www.dragonflyfund.org/ This series intends to publish studies promoted by IDF and to facilitate cost-efficient and ra- pid dissemination of odonatological data.. Editorial Work: Martin Schorr Layout: Martin Schorr IDF-home page: Holger Hunger Indexed: Zoological Record, Thomson Reuters, UK Printing: Colour Connection GmbH, Frankfurt Impressum: Publisher: International Dragonfly Fund e.V., Schulstr. 7B, 54314 Zerf, Germany. E-mail: [email protected] and Verlag Natur in Buch und Kunst, Dieter Prestel, Beiert 11a, 53809 Ruppichteroth, Germany (Bestelladresse für das Druckwerk). E-mail: [email protected] Responsible editor: Martin Schorr Cover picture: Calopteryx virgo (left) and Calopteryx splendens (right), Finland Photographer: Sami Karjalainen Published 09.02.2016 Catalogue of individuals commemorated in the scientific names of extant dragonflies, including lists of all available eponymous species-group and genus-group names – Revised edition Matti Hämäläinen Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, the Netherlands E-mail: [email protected]; [email protected] Abstract A catalogue of 1290 persons commemorated in the scientific names of extant dra- gonflies (Odonata) is presented together with brief biographical information for each entry, typically the full name and year of birth and death (in case of a deceased person).