Chapter 1 General Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Symbiodinium) in Scleractinian Corals from Tropical Reefs in Southern Hainan
Journal of Systematics and Evolution 49 (6): 598–605 (2011) doi: 10.1111/j.1759-6831.2011.00161.x Research Article Low genetic diversity of symbiotic dinoflagellates (Symbiodinium) in scleractinian corals from tropical reefs in southern Hainan Island, China 1,2Guo-Wei ZHOU 1,2Hui HUANG∗ 1(Key Laboratory of Marine Bio-resources Sustainable Utilization, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China) 2(Tropical Marine Biological Research Station in Hainan, Chinese Academy of Sciences, Sanya 572000, China) Abstract Endosymbiotic dinoflagellates in the genus Symbiodinium are among the most abundant and important group of photosynthetic protists found in coral reef ecosystems. In order to further characterize this diversity and compare with other regions of the Pacific, samples from 44 species of scleractinian corals representing 20 genera and 9 families, were collected from tropical reefs in southern Hainan Island, China. Denaturing gradient gel electrophoresis fingerprinting of the ribosomal internal transcribed spacer 2 identified 11 genetically distinct Symbiodinium types that have been reported previously. The majority of reef-building coral species (88.6%) harbored only one subcladal type of symbiont, dominated by host-generalist C1 and C3, and was influenced little by the host’s apparent mode of symbiont acquisition. Some species harbored more than one clade of Symbiodinium (clades C, D) concurrently. Although geographically isolated from the rest of the Pacific, the symbiont diversity in southern Hainan Island was relatively low and similar to both the Great Barrier Reef and Hawaii symbiont assemblages (dominated by clade C Symbiodinium). These results indicate that a specialist symbiont is not a prerequisite for existence in remote and isolated areas, but additional work in other geographic regions is necessary to test this idea. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Settlement of Larvae from Four Families of Corals in Response to a Crustose Coralline Alga and Its Biochemical Morphogens Taylor N
www.nature.com/scientificreports OPEN Settlement of larvae from four families of corals in response to a crustose coralline alga and its biochemical morphogens Taylor N. Whitman1,2, Andrew P. Negri 1, David G. Bourne 1,2 & Carly J. Randall 1* Healthy benthic substrates that induce coral larvae to settle are necessary for coral recovery. Yet, the biochemical cues required to induce coral settlement have not been identifed for many taxa. Here we tested the ability of the crustose coralline alga (CCA) Porolithon onkodes to induce attachment and metamorphosis, collectively termed settlement, of larvae from 15 ecologically important coral species from the families Acroporidae, Merulinidae, Poritidae, and Diploastreidae. Live CCA fragments, ethanol extracts, and hot aqueous extracts of P. onkodes induced settlement (> 10%) for 11, 7, and 6 coral species, respectively. Live CCA fragments were the most efective inducer, achieving over 50% settlement for nine species. The strongest settlement responses were observed in Acropora spp.; the only non-acroporid species that settled over 50% were Diploastrea heliopora, Goniastrea retiformis, and Dipsastraea pallida. Larval settlement was reduced in treatments with chemical extracts compared with live CCA, although high settlement (> 50%) was reported for six acroporid species in response to ethanol extracts of CCA. All experimental treatments failed (< 10%) to induce settlement in Montipora aequituberculata, Mycedium elephantotus, and Porites cylindrica. Individual species responded heterogeneously to all treatments, suggesting that none of the cues represent a universal settlement inducer. These results challenge the commonly-held notion that CCA ubiquitously induces coral settlement, and emphasize the critical need to assess additional cues to identify natural settlement inducers for a broad range of coral taxa. -

The Reproduction of the Red Sea Coral Stylophora Pistillata
MARINE ECOLOGY PROGRESS SERIES Vol. 1, 133-144, 1979 - Published September 30 Mar. Ecol. Prog. Ser. The Reproduction of the Red Sea Coral Stylophora pistillata. I. Gonads and Planulae B. Rinkevich and Y.Loya Department of Zoology. The George S. Wise Center for Life Sciences, Tel Aviv University. Tel Aviv. Israel ABSTRACT: The reproduction of Stylophora pistillata, one of the most abundant coral species in the Gulf of Eilat, Red Sea, was studied over more than two years. Gonads were regularly examined using histological sections and the planula-larvae were collected in situ with plankton nets. S. pistillata is an hermaphroditic species. Ovaries and testes are situated in the same polyp, scattered between and beneath the septa and attached to them by stalks. Egg development starts in July preceding the spermaria, which start to develop only in October. A description is given on the male and female gonads, their structure and developmental processes. During oogenesis most of the oocytes are absorbed and usually only one oocyte remains in each gonad. S. pistillata broods its eggs to the planula stage. Planulae are shed after sunset and during the night. After spawning, the planula swims actively and changes its shape frequently. A mature planula larva of S. pistillata has 6 pairs of complete mesenteries (Halcampoides stage). However, a wide variability in developmental stages exists in newly shed planulae. The oral pole of the planula shows green fluorescence. Unique organs ('filaments' and 'nodules') are found on the surface of the planula; -

Pleistocene Reefs of the Egyptian Red Sea: Environmental Change and Community Persistence
Pleistocene reefs of the Egyptian Red Sea: environmental change and community persistence Lorraine R. Casazza School of Science and Engineering, Al Akhawayn University, Ifrane, Morocco ABSTRACT The fossil record of Red Sea fringing reefs provides an opportunity to study the history of coral-reef survival and recovery in the context of extreme environmental change. The Middle Pleistocene, the Late Pleistocene, and modern reefs represent three periods of reef growth separated by glacial low stands during which conditions became difficult for symbiotic reef fauna. Coral diversity and paleoenvironments of eight Middle and Late Pleistocene fossil terraces are described and characterized here. Pleistocene reef zones closely resemble reef zones of the modern Red Sea. All but one species identified from Middle and Late Pleistocene outcrops are also found on modern Red Sea reefs despite the possible extinction of most coral over two-thirds of the Red Sea basin during glacial low stands. Refugia in the Gulf of Aqaba and southern Red Sea may have allowed for the persistence of coral communities across glaciation events. Stability of coral communities across these extreme climate events indicates that even small populations of survivors can repopulate large areas given appropriate water conditions and time. Subjects Biodiversity, Biogeography, Ecology, Marine Biology, Paleontology Keywords Coral reefs, Egypt, Climate change, Fossil reefs, Scleractinia, Cenozoic, Western Indian Ocean Submitted 23 September 2016 INTRODUCTION Accepted 2 June 2017 Coral reefs worldwide are threatened by habitat degradation due to coastal development, 28 June 2017 Published pollution run-off from land, destructive fishing practices, and rising ocean temperature Corresponding author and acidification resulting from anthropogenic climate change (Wilkinson, 2008; Lorraine R. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
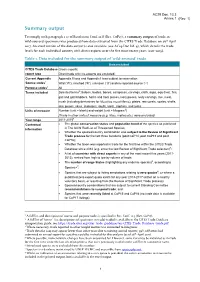
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of -

5. Putra Shallow Water Hardcorel Revised
Aceh Journal of Animal Science (2019) 4(2): 89-98 DOI: 10.13170/ajas.4.2.14571 Printed ISSN 2502-9568 Electronic ISSN 2622-8734 RESEARCH PAPER Shallow-water hard corals (Hexacorallia: Scleractinia) from Bangka Belitung Islands Waters, Indonesia Singgih Afifa Putra1*, Helmy Akbar2, Indra Ambalika Syari3 1Center for Development, Empowerment of Educators and Education Officer of The Marine and Fisheries Information and Communication Technology, Gowa, Sulawesi Selatan, Indonesia; 2Department of Marine Sciences, Universitas Mulawarman, Samarinda, Kalimantan Timur, Indonesia; 3Department of Marine Sciences, Universitas Bangka Belitung, Bangka, Kep. Bangka Belitung, Indonesia *Corresponding author’s email: [email protected] Received : 07 September 2019 Accepted : 30 October 2019 ABSTRACT Bangka Belitung Islands (Sumatra, Indonesia) has various coastal resources, e.g., coral reefs, seagrass beds, mangrove forests. However, the coral community has been threatened by anthropogenic activities, i.e., tin mining and illegal tin mining. Threatened species assessment is important for mitigation of coral losses and management. The ojective of the present study was to examine the status of Scleractinian corals in Bangka Belitung Islands, Indonesia. A line intercept transect was performed for the coral reef survey. Live and dead coral cover were recorded in the three locations. Corals species were identified following taxonomic revisions. The results showed that there were 142 species of Scleractinian corals recorded from Bangka Belitung Islands. Of these, 22 species are the new report from the areas of the the eastern part of Belitung Island. Family of Merulinidae, Acroporidae, and Poritidae were predominant group in this region. It is concluded that the condition of the coral reef ecosystem in the Belitung Islands is relatively good, but fair in Gaspar Strait and Bangka Island. -

Review of Corals from Fiji, Haiti, Solomon Islands and Tonga
UNEP-WCMC technical report Review of corals from Fiji, Haiti, Solomon Islands and Tonga (Coral species subject to EU decisions where identification to genus level is acceptable for trade purposes) (Version edited for public release) Review of corals from Fiji, Haiti, Solomon Islands and Tonga (coral 2 species subject to EU decisions where identification to genus level is acceptable for trade purposes) Prepared for The European Commission, Directorate General Environment, Directorate E - Global & Regional Challenges, LIFE ENV.E.2. – Global Sustainability, Trade & Multilateral Agreements, Brussels, Belgium Prepared March 2015 Copyright European Commission 2015 Citation UNEP-WCMC. 2015. Review of corals from Fiji, Haiti, Solomon Islands and Tonga (coral species subject to EU decisions where identification to genus level is acceptable for trade purposes). UNEP-WCMC, Cambridge. The UNEP World Conservation Monitoring Centre (UNEP-WCMC) is the specialist biodiversity assessment of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organization. The Centre has been in operation for over 30 years, combining scientific research with policy advice and the development of decision tools. We are able to provide objective, scientifically rigorous products and services to help decision- makers recognize the value of biodiversity and apply this knowledge to all that they do. To do this, we collate and verify data on biodiversity and ecosystem services that we analyze and interpret in comprehensive assessments, making the results available in appropriate forms for national and international level decision-makers and businesses. To ensure that our work is both sustainable and equitable we seek to build the capacity of partners where needed, so that they can provide the same services at national and regional scales. -

Bulletin of the United States Fish Commission
THE STONY CORALS OE THE PORTO RICAN WATERS. BY T: WAYLAND VAUGHAN. CONTENTS. Page. Page. Introduction 291 Discussion of Species—Continued. Table of Species 291 Class Anthozoa—Continued. Discussion of Species 292-318 Order Actiniae—Continued. Class Anthozoa 292 Family Favidae—Continued. Order Actiniae 292 Genus Manicina 305 Family Caryophyllidae 292 Manieina areolata 305 Genus Caryophyllia 292 Genus Platygyra 305,306 Caryophyllia berteriana? 292 Platygyra viridis 306-308 Genus Paracyathus 292 Family Agaricidse 309 Paracyathus de filippii 292 Genus Siderastrea 309 Genus Oyathoceras 293 Siderastrea radians 309 Cyathoceras portoricensis 293 Siderastrea siderea 309, 310 Genus Deltocyathus 293 Genus Agaricia 310 Deltocyathus italicus 293 Agaricia elephantotus 310 Agaricia sp 310, 311 Family Oculinidae 294 Agaricia cailleti 311 Genus Oculina 294 Genus Diaseris 311 Oculina diffusa? var 294 “ Diaseris ” crispa 311 Family Stylophoridse 294 Family Isoporidae 312 Genus Axhelia 294 Genus Isopora 312 Axhelia asperula 294 Isopora muricata 312, 313 Axhelia mirabilis 295 Isopora muricata ss. (== cervicornis) 313 Family Eusmilidae 295 Isopora muricata forma prolifera 313 Genus Meandrina 295 Isopora muricata forma palmata 313, 314 Meandrina maeandrites 296,297 Family Poritidae 314 Family Astrangidae 298 Genus Porites 314 Genus Cladocora 298 Porites porites 314-316 Cladocora arbuscula 298 Porites porites forma clavaria 316 Cladocora debilis 298 Porites porites forma furcata 316 Genus Astrangia 298 Porites porites forma divaricata 316 Astrangia solitaria? var. portoricensis 298,299 Porites astreoides 317 Astrangia astreiformis 300 Porites astreoides forma a 317 Family Orbicellidae 300 Porites astreoides forma /3 318 Genus Orbicella 300 Class Hydrozoa 318 Orbicella acropora var 301 Order Hydrocoral linse 318 Family Favidae 302 Family Milleporidae 318 Genus Favia 303 Genus Millepora 318 Favia fragum 303, 304 Millepora alcicornis 318 290 . -

Final 83326-Wjze.Xps
World Journal of Zoology 9 (2): 93-100, 2014 ISSN 1817-3098 © IDOSI Publications, 2014 DOI: 10.5829/idosi.wjz.2014.9.2.83326 Threatened Scleractinian Corals of Andaman and Nicobar Islands, India 11Tamal Mondal, C. Raghunathan and 2K. Venkataraman 1Zoological Survey of India, Andaman and Nicobar Regional Centre, National Coral Reef Research Institute, Haddo, Port Blair-744 102, Andaman and Nicobar Islands, India 2Zoological Survey of India, Prani Vigyan Bhavan, M- Block, New Alipore, Kolkata-700 053, India Abstract: Conservation is the measure to safeguard any species against the depletion. Depending upon the natural and human threats, several species are under threat towards extinction. The International Union for Conservation of Nature (IUCN) was founded to compute the status of floral and faunal lives to protect them against degradation by means of several categorizations. Threatened species is the combination of three categories such as Critically Endangered, Endangered and Vulnerable which are very near to extinction stage. Depending upon five criterions 228 species of scleractinian corals were categorized under threatened species. Andaman and Nicobar Islands harbors 121 species of threatened corals comprising of 8.26% Endangered (EN) and 90.90% Vulnerable (VU) species. Only one species of scleractinian coral under Critically Endangered (CR) category was reported from Andaman and Nicobar Islands. Among them, 44.62% species are common in occurrence and distribution in Andaman and Nicobar Islands which implies the enriched marine biodiversity of these areas. Key words: Conservation Threatened Species Endangered Vulnerable Andaman and Nicobar Islands INTRODUCTION contributing a lot for sustainable development of the world from biological, ecological, socio-economical etc.