FOOD ADDITIVES THAT CAUSE HYPERACTIVITY 102 & E102 Tartrazine Artificial Colouring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Technology Glossary
Glossary of Fish Technology Terms A Selection of Terms Compiled by Kevin J. Whittle and Peter Howgate Prepared under contract to the Fisheries Industries Division of the Food and Agriculture Organization of the United Nations 6 December 2000 Last updated: February 2002 Kevin J. Whittle 1 GLOSSARY OF FISH TECHNOLOGY TERMS [Words highlighted in bold in the text of an entry refer to another entry. Words in parenthesis are alternatives.] Abnormalities Attributes of the fish that are not found in the great majority of that kind of fish. For example: atypical shapes; overall or patchy discolorations of skin or of fillet; diseased conditions; atypical odours or flavours. Generally, the term should be used for peculiarities present in the fish at the time of capture or harvesting, or developing very soon after; peculiarities arising during processing should be considered as defects. Acetic acid Formal chemical name, ethanoic acid. An organic acid of formula CH3.COOH. It is the main component, 3-6%, other than water, of vinegar. Used in fish technology in preparation of marinades. Acid curing See Marinating Actomyosin A combination of the two main proteins, actin and myosin, present in all muscle tissues. Additive A chemical added to a food to affect its properties. Objectives of including additives in a product include: increased stability during storage; inhibition of growth of microorganisms or production of microbial toxins; prevention or reduction of formation of off-flavours; improved sensory properties, particularly colours and appearance, affecting acceptability to the consumer; improved properties related to preparation and processing of food, for example, ability to create stable foams or emulsions, or to stabilise or thicken sauces. -

Charles A. S. Hall
Charles A. S. Hall 354 Illick Hall SUNY College of Environmental Science and Forestry One Forestry Drive, Syracuse, NY 13210 (315) 470-6870 or 470-6743 (Secretary); 470-6934 (FAX) CURRICULUM VITAE EDUCATION B.A. Colgate University, Hamilton, NY, Biology 1965 (Advisor: Oran Stanley) M.S. Pennsylvania State University, Univ. Park PA, Zoology 1966 (Advisor: William Cooper) Ph.D. University of North Carolina, Chapel Hill NC, Zoology 1970 (Advisor: H. T. Odum) PROFESSIONAL POSITIONS (Post Ph.D.) 1970 - 1974 Research Associate, Staff Scientist II (half time), Department of Biology, Brookhaven National Laboratory, Upton, NY (Director: George Woodwell) 1975 - 1977 Research Scientist II (half-time), The Ecosystems Center, Marine Biological Laboratory, Woods Hole, MA (Director: George Woodwell) 1972 - 1985 Visiting Assistant Professor, Assistant Professor, Section of Ecology and Systematics, Cornell University, Ithaca, NY 1985 - 1987 Research Associate Professor, Biological Station and Department of Zoology, University of Montana, Yellow Bay and Missoula, MT 1987-1992 Associate Professor, SUNY College of Environmental Science and Forestry, Syracuse, NY 1992 - Professor, SUNY College of Environmental Science and Forestry, Syracuse, NY 2001 - ESF Foundation Distinguished Professor, SUNY College of Environmental Science and Forestry, Syracuse, NY PROFESSIONAL INTERESTS AND GOALS Systems Ecology: The application of integrative tools of science, including especially empirical simulation modeling, to the understanding and management of complex systems of nature and of people and nature. My principal focus throughout the diversity of projects represented herein is, and always has been, the examination of how organisms and societies invest energy in resource exploitation, and how such investments change as the quality of resources changes. -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5 -

Evaluation of Certain Food Additives
WHO Technical Report Series 1014 Evaluation of certain food additives Eighty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives The World Health Organization (WHO) was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for international health matters and public health. One of WHO’s constitutional functions is to provide objective and reliable information and advice in the field of human health, a responsibility that it fulfils in part through its extensive programme of publications. The Organization seeks through its publications to support national health strategies and address the most pressing public health concerns of populations around the world. To respond to the needs of Member States at all levels of development, WHO publishes practical manuals, handbooks and training material for specific categories of health workers; internationally applicable guidelines and standards; reviews and analyses of health policies, programmes and research; and state-of-the-art consensus reports that offer technical advice and recommendations for decision-makers. These books are closely tied to the Organization’s priority activities, encompassing disease prevention and control, the development of equitable health systems based on primary health care, and health promotion for individuals and communities. Progress towards better health for all also demands the global dissemination and exchange of information that draws on the knowledge and experience of all WHO Member States and the collaboration of world leaders in public health and the biomedical sciences. To ensure the widest possible availability of authoritative information and guidance on health matters, WHO secures the broad international distribution of its publications and encourages their translation and adaptation. -

Kids First Campaign
SUBMISSION TO THE CHAIR OF FSANZ 10 MARCH 2009 REGARDING FOOD COLOURS Australian regulations on artificial colours are inconsistent with overseas use TABLE 1 Artificial colours permitted in Australia Code Name Banned or restricted in other countries 102* Tartrazine UK, EU, Norway 104* Quinoline Yellow UK, USA, Japan, EU 110* Sunset Yellow UK, EU 122* Azorubine, UK, EU, USA, Canada, Japan, Norway, Sweden Carmoisine 123 Amaranth USA 124* Ponceau, Brilliant UK, EU, USA, Norway, Finland Scarlet 127 Erythrosine Rarely used in the USA due to known hazards 129* Allura Red UK, EU 132 Indigotine 133 Brilliant Blue 142 Green S USA, many other countries 143 Fast Green FCF EU, some other countries 151 Brilliant Black USA, Canada, Finland, Japan, Norway 155 Brown HT USA, Austria, Belgium, Denmark, France, Germany, Norway, Sweden, Switzerland * Southampton Six colours. Due to constant changes, there may be minor inaccuracies in this table. • In the UK, the six artificial colours marked with an asterisk (the so called Southampton Six) are subject to a ‘voluntary phase out’ by the end of 2009. http://www.foodnavigator.com/Legislation/Ministers-on-board-with- Southampton-six-phase-out • Most of the big manufacturers in the UK are extending the voluntary ban to all artificial colours, including Brilliant Blue 133 – e.g. there was big publicity when artificially coloured blue Smarties were withdrawn in the UK due to health concerns about E133 in 2006 and when naturally coloured blue Smarties were introduced in 2008. 1 • By the end of 2009 in the EU foods containing the Southampton Six colours will have to carry the warning: "may have an adverse effect on activity and attention in children."http://www.europarl.europa.eu/news/expert/infopress_page/067- 33565-189-07-28-911-20080707IPR33563-07-07-2008-2008- false/default_en.htm Aussie kids most at risk from artificial colours 123 and 155 says FSANZ survey in December 2008. -

New Reference Materials for Residue Analysis
New ref erence materials f or residue analysis Pesticides and metabolites • VOCs • PA Hs • Dy es • Ve te r in a ry d r u g s • P h a r m a c e u t i c a l c o n t a m i n a n t s • R e g u l a t o r y m i x e s • H ydrocarbons • Petrochemicals • ydrocarbons LGC Quality - I S O G uide 34 • G M P/ G L P • I S O 9001 • I S O/ I E C 17 025 • I S O/ I E C 17 043 Introducing over 600 new products from Dr. Ehrenstorfer Welcome to the Dr. Ehrenstorfer catalogue supplement, where you will fi nd new products including: Mixtures for popular ASTM and EPA regulatory methods New pesticide and pesticide metabolite standards Stable isotope labelled standards A wider range of veterinary and pharmaceutical residue standards including marker metabolites Extractable Petroleum Hydrocarbon (EPH) mixtures and other hydrocarbon reference materials These new products add to the comprehensive range of 8000 Dr. Ehrenstorfer standards. Dr. Siegmund Ehrenstorfer pioneered the production of pesticide reference substances in the 1960s. Today, the portfolio has expanded and adapted to meet changing regulations and technology. Order online at: www.lgcstandards.com Prefer to speak to someone? Call your local sales offi ce, contact details are on the back cover. Contents 1 Dyes 3 Food related compounds 4 Hydrocarbons and Petrochemicals 11 Pesticides 28 Pharmaceutical and Veterinary compounds 36 Phenol and aromatic compounds 39 Plasticizers 41 Polycyclic aromatic compounds 43 Polyhalogenated phenyls 44 Regulatory products 58 Volatile organic compounds 60 Other classes 65 -
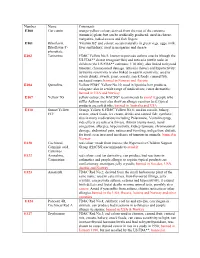
Number Name Comments E100 Cur Cumin
Number Name Comments E100 Cur cumin orange-yellow colour; derived from the root of the curcuma (turmeric) plant, but can be artificially produced; used in cheese, margarine, baked sweets and fish fingers E101 Riboflavin, 'Vitamin B2' and colour; occurs naturally in green vegs, eggs, milk, Riboflavin-5'- liver and kidney; used in margarine and cheese phosphate E102 Tartrazine FD&C Yellow No.5; known to provoke asthma attacks (though the US FDA** do not recognise this) and urticaria (nettle rash) in children (the US FDA** estimates 1:10 000); also linked to thyroid tumours, chromosomal damage, urticaria (hives) and hyperactivity; tartrazine sensitivity is also linked to aspirin sensitivity; used to colour drinks, sweets, jams, cereals, snack foods, canned fish, packaged soups; banned in Norway and Austria E104 Quinoline Yellow FD&C Yellow No.10; used in lipsticks hair products, colognes; also in a wide range of medications; cause dermatitis; banned in USA and Norway E107 Yellow 7G yellow colour; the HACSG* recommends to avoid it; people who suffer Asthma may also show an allergic reaction to it; typical products are soft drinks; banned in Australia and USA E110 Sunset Yellow Orange Yellow S FD&C Yellow No.6; used in cereals, bakery, FCF sweets, snack foods, ice cream, drinks and canned fish; synthetic; also in many medications including Polaramine, Ventolin syrup; side effects are urticaria (hives), rhinitis (runny nose), nasal congestion, allergies, hyperactivity, kidney tumours, chromosomal damage, abdominal pain, nausea and vomiting, -

Food & Beverage Litigation Update
ISSUE 346 | APRIL 23, 2010 FOOD & BEVERAGE LITIGATION UPDATE CONTENTS Legislation, Regulations and Standards FDA Action on Sodium Intake Among IOM Recommendations. 1 Consumer & Organic Interests Challenge U.S. Draft Position on Codex Labeling for GM Products. .2 LEGISLATION, REGULATIONS AND STANDARDS Organics Watchdog Calls on USDA, FTC to Stop Use of “Organic” in FDA Action on Sodium Intake Among IOM Recommendations Company Names. 3 FDA Seeks Information on Pathogens At the request of Congress, the Institute of Medicine (IOM) has prepared and in Spices. .4 released a report titled “Strategies to Reduce Sodium Intake in the United States.” Dietary Guidelines Advisory Committee Plans Final Meeting. 4 Starting from the premise that “Americans consume unhealthy amounts of EU Agrees to Remove U.S. Rice sodium in their food,” which puts some 100,000 at risk of premature death from Import Restrictions. 4 conditions related to high blood pressure, the report calls for the Food and Drug EFSA Reevaluates Scientific Opinion for Administration (FDA) to “set mandatory national standards for the sodium content Three Food Colorings. 5 in foods—not banning outright the addition of salt to foods but beginning the Danone Withdraws Requests That EFSA process of reducing excess sodium in processed foods and menu items to a safer Approve Health Claims for Yogurt Products. .5 level.” According to IOM, this reduction must be carried out gradually so consumers’ Canadian Government Considers tastes could adjust, a process that could take up to 10 years. Changes to “Made in Canada” Label . 5 Draft Nanotech Policy Framework for Other recommendations include an FDA modification of the generally recognized California Scheduled for Discussion as safe (GRAS) status of sodium-containing compounds added to processed and Comment. -
Guidance on Food Additives, Revision 2, 2015
SUMMARY SUMMARY | INTRODUCTION | CHAPTER 1 | CHAPTER 2 | CHAPTER 3 | CHAPTER 4 | CHAPTER 5 | CHAPTER 6 2, 2015 | REFERENCES evision R Guidance on Food Additives Food Safety Authority of Ireland | Guidance on Food Additives, Revision 2, 2015 PREFACE This guidance document provides an overview of the European Union and National legislation related to additives in food, together with guidance on its interpretation. This document does not purport to be comprehensive or to be a legal interpretation or to constitute legal or other professional advice. Unless otherwise stated, the definitions and terminology used in this report relate to this document only. References to the applicable legislation are valid, to the best knowledge of the Food Safety Authority of Ireland (FSAI), at the time of publication. Advances in scientific knowledge and changes in legislation can be expected in the future that will necessitate the updating of this document. Further information on all of the legislation covered by this guidance or introduced subsequent to its date of publication (November, 2015) is available from the fsai website. November, 2015 Published by: Food Safety Authority of Ireland Abbey Court Lower Abbey Street Dublin 1 DO1 W2H4 Tel: 01 817 1300 Email: [email protected] Website: www.fsai.ie ©FSAI 2015 Applications for reproduction should be made to the FSAI Information Unit ISBN 1-904465-72-2 Food Safety Authority of Ireland | Guidance on Food Additives, Revision 2, 2015 1 Table of Contents SUMMARY . 3 INTRODUCTION ........................................................................................ 5 CHAPTER 1. FOOD ADDITIVES, FUNCTIONAL CLASSES, LISTS OF AUTHORISED FOOD ADDITIVES AND THE FOOD CATEGORIES TO WHICH THEY MAY BE ADDED . -

B Commission Regulation (Eu)
2011R1129 — EN — 21.11.2013 — 001.001 — 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives (Text with EEA relevance) (OJ L 295, 12.11.2011, p. 1) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 1152/2013 of 19 November 2013 L 311 1 20.11.2013 2011R1129 — EN — 21.11.2013 — 001.001 — 2 ▼B COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (1 ), and in particular Article 10, Article 30(1) and Article 30(5) thereof, Whereas: (1) Regulation (EC) No 1333/2008 provides for the establishment of a Union list of food additives approved for use in foods and their conditions of use. (2) Food additives which are currently permitted for use in foods under European Parliament and Council Directive 94/35/EC of 30 June 1994 on sweeteners for use in foodstuffs (2 ), European Parliament and Council Directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs (3 ) and European Parliament and Council Directive 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners (4 ), should be included in Annex II to Regulation (EC) No 1333/2008 after a review of their compliance with Articles 6, 7 and 8 thereof. -

542 Final REPORT from the COMMISSION on Dietary Food Addit
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 01.10.2001 COM(2001) 542 final REPORT FROM THE COMMISSION on Dietary Food Additive Intake in the European Union REPORT FROM THE COMMISSION on Dietary Food Additive Intake in the European Union TABLE OF CONTENTS Executive Summary ...............................................................................................................3 1 Introduction..............................................................................................................4 2 Background ..............................................................................................................5 3 The monitoring task..................................................................................................7 3.1 Additives excluded from the monitoring task and further examination:.....................7 3.2 Additives subject to tier-1 screening .........................................................................7 3.3 Additives subject to tier-2 screening .........................................................................8 3.4 Additives subject to tier-3 screening .........................................................................8 4 The monitoring data..................................................................................................8 4.1 Instructions for reporting the monitoring data ...........................................................8 4.2 The type of monitoring data obtained........................................................................9 4.2.1 -

Food Additives Data Book
Food Additives Data Book Food Additives Data Book Edited by Jim Smith and Lily Hong-Shum Blackwell Science © 2003 by Blackwell Science Ltd, First published 2003 by Blackwell Science Ltd a Blackwell Publishing Company Editorial Offices: Library of Congress Osney Mead, Oxford OX2 0EL, UK Cataloging-in-Publication Data Tel: +44 (0)1865 206206 is available Blackwell Science, Inc., 350 Main Street, Malden, MA 02148-5018, USA 0-632-06395-5 Tel: +1 781 388 8250 Iowa State Press, a Blackwell Publishing A catalogue record for this title is available Company, 2121 State Avenue, Ames, Iowa from the British Library 50014-8300, USA Tel: +1 515 292 0140 Set in 9 on 11pt Times New RomanPS Blackwell Publishing Asia Pty Ltd, SNP Best-set Typesetter Ltd., Hong Kong 550 Swanston Street, Carlton South, http://www.sparks.co.uk Victoria 3053, Australia Printed and bound in Great Britain by Tel: +61 (0)3 9347 0300 TJ International Limited, Padstow Blackwell Wissenschafts Verlag, Kurfürstendamm 57, 10707 Berlin, Germany For further information on Tel: +49 (0)30 32 79 060 Blackwell Science, visit our website: www.blackwell-science.com The right of the Author to be identified as the Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.