Food & Beverage Litigation Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Technology Glossary
Glossary of Fish Technology Terms A Selection of Terms Compiled by Kevin J. Whittle and Peter Howgate Prepared under contract to the Fisheries Industries Division of the Food and Agriculture Organization of the United Nations 6 December 2000 Last updated: February 2002 Kevin J. Whittle 1 GLOSSARY OF FISH TECHNOLOGY TERMS [Words highlighted in bold in the text of an entry refer to another entry. Words in parenthesis are alternatives.] Abnormalities Attributes of the fish that are not found in the great majority of that kind of fish. For example: atypical shapes; overall or patchy discolorations of skin or of fillet; diseased conditions; atypical odours or flavours. Generally, the term should be used for peculiarities present in the fish at the time of capture or harvesting, or developing very soon after; peculiarities arising during processing should be considered as defects. Acetic acid Formal chemical name, ethanoic acid. An organic acid of formula CH3.COOH. It is the main component, 3-6%, other than water, of vinegar. Used in fish technology in preparation of marinades. Acid curing See Marinating Actomyosin A combination of the two main proteins, actin and myosin, present in all muscle tissues. Additive A chemical added to a food to affect its properties. Objectives of including additives in a product include: increased stability during storage; inhibition of growth of microorganisms or production of microbial toxins; prevention or reduction of formation of off-flavours; improved sensory properties, particularly colours and appearance, affecting acceptability to the consumer; improved properties related to preparation and processing of food, for example, ability to create stable foams or emulsions, or to stabilise or thicken sauces. -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5 -
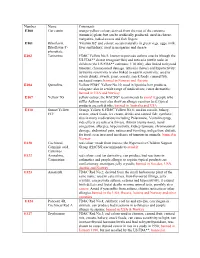
Number Name Comments E100 Cur Cumin
Number Name Comments E100 Cur cumin orange-yellow colour; derived from the root of the curcuma (turmeric) plant, but can be artificially produced; used in cheese, margarine, baked sweets and fish fingers E101 Riboflavin, 'Vitamin B2' and colour; occurs naturally in green vegs, eggs, milk, Riboflavin-5'- liver and kidney; used in margarine and cheese phosphate E102 Tartrazine FD&C Yellow No.5; known to provoke asthma attacks (though the US FDA** do not recognise this) and urticaria (nettle rash) in children (the US FDA** estimates 1:10 000); also linked to thyroid tumours, chromosomal damage, urticaria (hives) and hyperactivity; tartrazine sensitivity is also linked to aspirin sensitivity; used to colour drinks, sweets, jams, cereals, snack foods, canned fish, packaged soups; banned in Norway and Austria E104 Quinoline Yellow FD&C Yellow No.10; used in lipsticks hair products, colognes; also in a wide range of medications; cause dermatitis; banned in USA and Norway E107 Yellow 7G yellow colour; the HACSG* recommends to avoid it; people who suffer Asthma may also show an allergic reaction to it; typical products are soft drinks; banned in Australia and USA E110 Sunset Yellow Orange Yellow S FD&C Yellow No.6; used in cereals, bakery, FCF sweets, snack foods, ice cream, drinks and canned fish; synthetic; also in many medications including Polaramine, Ventolin syrup; side effects are urticaria (hives), rhinitis (runny nose), nasal congestion, allergies, hyperactivity, kidney tumours, chromosomal damage, abdominal pain, nausea and vomiting, -
Guidance on Food Additives, Revision 2, 2015
SUMMARY SUMMARY | INTRODUCTION | CHAPTER 1 | CHAPTER 2 | CHAPTER 3 | CHAPTER 4 | CHAPTER 5 | CHAPTER 6 2, 2015 | REFERENCES evision R Guidance on Food Additives Food Safety Authority of Ireland | Guidance on Food Additives, Revision 2, 2015 PREFACE This guidance document provides an overview of the European Union and National legislation related to additives in food, together with guidance on its interpretation. This document does not purport to be comprehensive or to be a legal interpretation or to constitute legal or other professional advice. Unless otherwise stated, the definitions and terminology used in this report relate to this document only. References to the applicable legislation are valid, to the best knowledge of the Food Safety Authority of Ireland (FSAI), at the time of publication. Advances in scientific knowledge and changes in legislation can be expected in the future that will necessitate the updating of this document. Further information on all of the legislation covered by this guidance or introduced subsequent to its date of publication (November, 2015) is available from the fsai website. November, 2015 Published by: Food Safety Authority of Ireland Abbey Court Lower Abbey Street Dublin 1 DO1 W2H4 Tel: 01 817 1300 Email: [email protected] Website: www.fsai.ie ©FSAI 2015 Applications for reproduction should be made to the FSAI Information Unit ISBN 1-904465-72-2 Food Safety Authority of Ireland | Guidance on Food Additives, Revision 2, 2015 1 Table of Contents SUMMARY . 3 INTRODUCTION ........................................................................................ 5 CHAPTER 1. FOOD ADDITIVES, FUNCTIONAL CLASSES, LISTS OF AUTHORISED FOOD ADDITIVES AND THE FOOD CATEGORIES TO WHICH THEY MAY BE ADDED . -

B Commission Regulation (Eu)
2011R1129 — EN — 21.11.2013 — 001.001 — 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives (Text with EEA relevance) (OJ L 295, 12.11.2011, p. 1) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 1152/2013 of 19 November 2013 L 311 1 20.11.2013 2011R1129 — EN — 21.11.2013 — 001.001 — 2 ▼B COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (1 ), and in particular Article 10, Article 30(1) and Article 30(5) thereof, Whereas: (1) Regulation (EC) No 1333/2008 provides for the establishment of a Union list of food additives approved for use in foods and their conditions of use. (2) Food additives which are currently permitted for use in foods under European Parliament and Council Directive 94/35/EC of 30 June 1994 on sweeteners for use in foodstuffs (2 ), European Parliament and Council Directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs (3 ) and European Parliament and Council Directive 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners (4 ), should be included in Annex II to Regulation (EC) No 1333/2008 after a review of their compliance with Articles 6, 7 and 8 thereof. -

FOOD ADDITIVES THAT CAUSE HYPERACTIVITY 102 & E102 Tartrazine Artificial Colouring
FOOD ADDITIVES THAT CAUSE HYPERACTIVITY 102 & E102 Tartrazine Artificial colouring. Common name for uncertified FD&C Yellow; No.5 Cl Acid Yellow; Acid Yellow 23; Cl Food Yellow 4; Coal Tar Dye;; Pyrazolone Dye. Tartrazine is an Aromatic Hydrocarbon. Causes cancer. The FDA states that over 100,000 people are allergic to tartrazine. U.K. studies have shown that 79% of hyperactive children are allergic to tartrazine. It is believed to cause allergic reactions in 15% of the total population. Known effects are urticaria, asthma, altered states of perception and behaviour, uncontrolled hyper agitation and confusion. Known reactions are rhinitis (hay fever), bronchospasms (breathing problems), blurred vision and purple patches on the skin. It may also cause wakefulness in young children at night. Tartrazine is known to inhibit zinc metabolism (zinc is required in over 200 enzyme systems in the body). It is an active ingredient in herbicides used to kill aquatic weeds. In 1986 the ‘British Institute of Mental Handicap’ linked it with aggressive behaviour in children. Prevents the action of an enzyme that breaks down many potentially harmful toxins formed during digestion. Tartrazine is one of four Azo dyes known to interfere with the digestive enzymes 1-Amylase, Pepsin and Trypsin. The British Medical Journal, the “Lancet”, lists these problems associated with tartrazine:- Allergies; Thyroid Tumours; Lymphocytic Lymphomas, Chromosomal Damage; Trigger for Asthma; Urticaria; Hyperactivity. As an Azo Dye, it is implicated in bladder cancer, liver cancer and sarcomas. (In hair dyes, tartrazine is absorbed through the skin.) Tartrazine is completely banned from foods in Norway and Finland and is heavily restricted in Austria, Sweden and Germany. -

Food Additives Data Book
Food Additives Data Book Food Additives Data Book Edited by Jim Smith and Lily Hong-Shum Blackwell Science © 2003 by Blackwell Science Ltd, First published 2003 by Blackwell Science Ltd a Blackwell Publishing Company Editorial Offices: Library of Congress Osney Mead, Oxford OX2 0EL, UK Cataloging-in-Publication Data Tel: +44 (0)1865 206206 is available Blackwell Science, Inc., 350 Main Street, Malden, MA 02148-5018, USA 0-632-06395-5 Tel: +1 781 388 8250 Iowa State Press, a Blackwell Publishing A catalogue record for this title is available Company, 2121 State Avenue, Ames, Iowa from the British Library 50014-8300, USA Tel: +1 515 292 0140 Set in 9 on 11pt Times New RomanPS Blackwell Publishing Asia Pty Ltd, SNP Best-set Typesetter Ltd., Hong Kong 550 Swanston Street, Carlton South, http://www.sparks.co.uk Victoria 3053, Australia Printed and bound in Great Britain by Tel: +61 (0)3 9347 0300 TJ International Limited, Padstow Blackwell Wissenschafts Verlag, Kurfürstendamm 57, 10707 Berlin, Germany For further information on Tel: +49 (0)30 32 79 060 Blackwell Science, visit our website: www.blackwell-science.com The right of the Author to be identified as the Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. -

Food Colorants: Their Past, Present, and Future
This is a repository copy of Food colorants: their past, present, and future. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/126336/ Version: Accepted Version Article: Coultate, T and Blackburn, RS orcid.org/0000-0001-6259-3807 (2018) Food colorants: their past, present, and future. Coloration Technology, 134 (3). pp. 165-186. ISSN 1472-3581 https://doi.org/10.1111/cote.12334 (c) 2018 The Authors. Coloration Technology (c) 2018 Society of Dyers and Colourists. This is the peer reviewed version of the following article: Food colorants: their past, present, and future Coultate, T and Blackburn, RS, Coloration Technology. which has been published in final form at https://doi.org/10.1111/cote.12334. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Food colorants: their past, present, and future Tom Coultate* Leighton Buzzard, Bedfordshire, LU7 2RW; *e-mail: [email protected] Richard S. -

Essential Guide to Food Additives
ESSENTIAL GUIDE TO FOOD ADDITIVES Third Edition Revised by Victoria Emerton and Eugenia Choi This edition first published 2008 by Leatherhead Publishing a division of Leatherhead Food International Ltd Randalls Road, Leatherhead, Surrey KT22 7RY, UK and Royal Society of Chemistry Thomas Graham House, Science Park, Milton Road, Cambridge, CB4 0WF, UK URL: http://www.rsc.org Registered Charity No. 207890 Third Edition 2008 ISBN No: 978-1-905224-50-0 A catalogue record of this book is available from the British Library © 2008 Leatherhead Food International Ltd The contents of this publication are copyright and reproduction in whole, or in part, is not permitted without the written consent of the Chief Executive of Leatherhead Food International Limited. Leatherhead Food International Limited uses every possible care in compiling, preparing and issuing the information herein given but accepts no liability whatsoever in connection with it. All rights reserved Apart from any fair dealing for the purposes of research or private study, or criticism or review as permitted under the terms of the UK Copyright, Designs and Patents Act, 1988, this publication may not be reproduced, stored or transmitted, in any form or by any means, without the prior permission in writing of the Chief Executive of Leatherhead Food International Ltd, or in the case of reprographic reproduction only in accordance with the terms of the licences issued by the Copyright Licencing Agency in the UK, or in accordance with the terms of the licences issued by the appropriate Reproduction Rights Organization outside the UK. Enquiries concerning reproduction outside the terms stated here should be sent to Leatherhead Food International Ltd at the address printed on this page. -

COLOURS E Numbers
COLOURS E Numbers (100-181) NUMBER NAME COMMENTS Orange-yellow colour; derived from the root of the curcuma plant, but can be 100 artificially produced; used in cheese, margarine, baked sweets and fish fingers It has Curcumin E100@ beneficial effect on the blood sugar in diabetics. It can increase the liver's secretion of bile and protect the liver from toxic substances. 101 Riboflavin, Riboflavin - 5'- 'Vitamin B2' and colour; occurs naturally in green vegetables, eggs, milk, liver and E101@ [phosphate] kidney; used in margarine and cheese. FD&C Yellow No:5; CI Acid Yellow23, CI Food Yellow 4. Coal tar dye. Polycyclic Aromatic Hydrocarbon. Cancer probability. Known to provoke asthma attacks (though the US FDA** do not recognise this) and urticaria (nettle rash) in children (the US FDA** estimates 1:10 000), altered states of perception and behaviour, uncontrolled 102 Tartrazine hyper agitation and confusion; wakefulness in young children. Is known to inhibit zinc E102 metabolism and interfere with digestive enzymes. Tartrazine sensitivity is also linked to aspirin sensitivity; used to colour drinks, sweets, jams, cereals, snack foods, canned fish, packaged soups. Banned in Norway, Austria and Finland. Restricted use in Sweden and Germany. Alkanet Natural 'port-wine' colour from A. tinctoria plant. Listed in Australia in 1992. Banned 103 (Chrysoine resorcinol) in US in 1988. D&C Yellow No:10; used in lipsticks hair products, colognes; also in a wide range of 104 medications; It may cause asthma, rashes and hyperactivity. Aspirin sensitive people Quinoline Yellow E104 must avoid it. Banned in Japan, USA and Norway. E105 Fast Yellow AB Riboflavin - E106 Listed in Australia as 100 prior to 1992. -

10 Colorants
Pag e 651 10 Colorants J. H. VON ELBE University of Wisconsin—Madison, Madison, Wisconsin STEVEN J. SCHWARTZ* North Carolina State University, Raleigh, North Carolina 10.1. Introduction 651 10.2. Pigments in Animal and Plant Tissue 653 10.2.1. Heme Compounds 653 10.2.2. Chlorophyll 659 10.2.3. Carotenoids 673 10.2.4. Flavonoids and Other Phenols 681 10.2.5. Betalaines 697 10.3. Food Colorants 703 10.3.1. Regulatory Aspects 703 10.3.2. Properties of Certified Dyes 708 10.3.3. Use of Certified Dyes 711 10.3.4. Colors Exempt from Certification 713 10.3.5. Use of Colors Exempt from Certification 717 Bibliography 718 References 718 10.1 Introduction To understand colorants in foods some terms need to be defined. Color refers to human perception of colored materials—red, green, blue, etc. A colorant is any chemical, either natural or synthetic, that imparts color. Foods have color because of their ability to reflect or emit different quantities of energy at wavelengths able to stimulate the retina in the eye. The energy *Present affiliation: The Ohio State University, Columbus, Ohio. Pag e 652 range to which the eye is sensitive is referred to as visible light. Visible light, depending on an individual's sensitivity, encompasses wavelengths of approximately 380–770 nm. This range makes up a very small portion of the electromagnetic spectrum (Fig. 1). In addition to obvious colors (hues), black, white, and intermediate grays are also regarded as colors. Pigments are natural substances in cells and tissues of plants and animals that impart color. -

EU) No 1129/2011 Amending Annex II to Regulation (EC
12.11.2011 EN Official Journal of the European Union L 295/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives (Text with EEA relevance) THE EUROPEAN COMMISSION, placed on the market and used in foods under the conditions of use specified therein. The additives should be listed on the basis of the categories of food to which Having regard to the Treaty on the Functioning of the European they may be added. In order to facilitate the transfer and Union, to enhance transparency of the authorisation procedure, it is appropriate to develop a new food categorisation system which will form the basis of Annex II. Having regard to Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives ( 1), and in particular Article 10, (4) The established Codex Alimentarius General Standard for Article 30(1) and Article 30(5) thereof, Food Additives ( 5 ), food category system has been used as a starting point for developing the Union system. However, that system needs to be adapted to take into Whereas: account the specificity of the existing food additive auth orisations in the Union. Current sector specific Union provisions on foods have been taken into account. The (1) Regulation (EC) No 1333/2008 provides for the estab categories are created with the sole purpose of listing the lishment of a Union list of food additives approved for authorised additives and their conditions of use.