Chapter 1: Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barriers to Fish Passage in Nova Scotia the Evolution of Water Control Barriers in Nova Scotia’S Watershed
Dalhousie University- Environmental Science Barriers to Fish Passage in Nova Scotia The Evolution of Water Control Barriers in Nova Scotia’s Watershed By: Gillian Fielding Supervisor: Shannon Sterling Submitted for ENVS 4901- Environmental Science Honours Abstract Loss of connectivity throughout river systems is one of the most serious effects dams impose on migrating fish species. I examine the extent and dates of aquatic habitat loss due to dam construction in two key salmon regions in Nova Scotia: Inner Bay of Fundy (IBoF) and the Southern Uplands (SU). This work is possible due to the recent progress in the water control structure inventory for the province of Nova Scotia (NSWCD) by Nova Scotia Environment. Findings indicate that 586 dams have been documented in the NSWCD inventory for the entire province. The most common main purpose of dams built throughout Nova Scotia is for hydropower production (21%) and only 14% of dams in the database contain associated fish passage technology. Findings indicate that the SU is impacted by 279 dams, resulting in an upstream habitat loss of 3,008 km of stream length, equivalent to 9.28% of the total stream length within the SU. The most extensive amount of loss occurred from 1920-1930. The IBoF was found to have 131 dams resulting in an upstream habitat loss of 1, 299 km of stream length, equivalent to 7.1% of total stream length. The most extensive amount of upstream habitat loss occurred from 1930-1940. I also examined if given what I have learned about the locations and dates of dam installations, are existent fish population data sufficient to assess the impacts of dams on the IBoF and SU Atlantic salmon populations in Nova Scotia? Results indicate that dams have caused a widespread upstream loss of freshwater habitat in Nova Scotia howeverfish population data do not exist to examine the direct impact of dam construction on the IBoF and SU Atlantic salmon populations in Nova Scotia. -

Hydrodynamic Modelling for Flood Management in Bay of Fundy Dykelands”
Hydrodynamic Modelling for Flood Management in Bay of Fundy Dykelands By Michael Fedak A Thesis Submitted to Saint Mary’s University, Halifax, Nova Scotia in Partial Fulfillment of the Requirements for the Degree of Master of Science in Applied Science July 16, 2012, Halifax Nova Scotia © Michael Fedak, 2012 Approved: Dr. Danika van Proosdij Supervisor Department of Geography Approved: Dr. Ryan Mulligan External Examiner Department of Civil Engineering Queen’s University Approved: Dr. Timothy Webster Supervisory Committee Member COGS, NSCC Approved: Dr. Peter Secord Graduate Studies Representative Date: July 16, 2012 Abstract “Hydrodynamic Modelling for Flood Management in Bay of Fundy Dykelands” By Michael Fedak Storm surge in the coastal Bay of Fundy is a serious flood hazard. These lands are low-lying and adapted to farming through the use of coastal defences, namely dykes. Increasing rates of sea level rise due to climate change are expected to increase flood hazard in this area. In this thesis, flood risk to communities in the Avon River estuary of the Upper Bay of Fundy is investigated through computer based modelling and data management techniques. Flood variables from 14 possible storm surge scenarios (based on sea level rise predictions) were modelled using TUFLOW software. A GIS was used to create a database for simulation outputs and for the analysis of outputs. TUFLOW and a geographic information system (GIS) flood algorithm are compared .It was found that obstructions to flow controlled flooding and drainage and these features required the use of a hydrodynamic model to represent flows properly. July 16, 2012 2 Acknowledgements Funding Sources: • Atlantic Canada Adaptation Solutions Association part of the Natural Resources Canada Regional Adaptation Collaboratives (RAC) Project. -
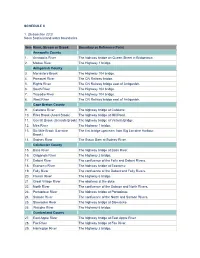
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

They Planted Well: New England Planters in Maritime Canada
They Planted Well: New England Planters in Maritime Canada. PLACES Acadia University, Wolfville, Nova Scotia, 9, 10, 12 Amherst Township, Nova Scotia, 124 Amherst, Nova Scotia, 38, 39, 304, 316 Andover, Maryland 65 Annapolis River, Nova Scotia, 22 Annapolis Township, Nova Scotia, 23, 122-123 Annapolis Valley, Nova Scotia, 10, 14-15, 107, 178 Annapolis County, Nova Scotia, 20, 24-26, 28-29, 155, 258 Annapolis Gut, Nova Scotia, 43 Annapolis Basin, Nova Scotia, 25 Annapolis-Royal (Port Royal-Annapolis), 36, 46, 103, 244, 251, 298 Atwell House, King's County, Nova Scotia, 253, 258-259 Aulac River, New Brunswick, 38 Avon River, Nova Scotia, 21, 27 Baie Verte, Fort, (Fort Lawrence) New Brunswick, 38 Barrington Township, Nova Scotia, 124, 168, 299, 315, Beaubassin, New Brunswick (Cumberland Basin), 36 Beausejour, Fort, (Fort Cumberland) New Brunswick, 17, 22, 36-37, 45, 154, 264, 277, 281 Beaver River, Nova Scotia, 197 Bedford Basin, Nova Scotia, 100 Belleisle, Annapolis County, Nova Scotia, 313 Biggs House, Gaspreau, Nova Scotia, 244-245 Blomidon, Cape, Nova Scotia, 21, 27 Boston, Massachusetts, 18, 30-31, 50, 66, 69, 76, 78, 81-82, 84, 86, 89, 99, 121, 141, 172, 176, 215, 265 Boudreau's Bank, (Starr's Point) Nova Scotia, 27 Bridgetown, Nova Scotia, 196, 316 Buckram (Ship), 48 Bucks Harbor, Maine, 174 Burton, New Brunswick, 33 Calkin House, Kings County, 250, 252, 259 Camphill (Rout), 43-45, 48, 52 Canning, Nova Scotia, 236, 240 Canso, Nova Scotia, 23 Cape Breton, Nova Scotia, 40, 114, 119, 134, 138, 140, 143-144 2 Cape Cod-Style House, 223 -

Upgrading the Avon River Causeway During Highway 101 Twinning
Upgrading the Avon River Causeway During Highway 101 Twinning Dr. Bob Pett, NSTIR Environmental Services Alexander Wilson, CBCL Limited Transportation and Infrastructure Renewal Partner with NS Agriculture 9.5 km 6 lanes PEI Moncton NB Northumberland Strait Petitcodiac River NS Chignecto Bay Minas Basin Bay of Fundy Avon River Windsor Fundy Tides Salty- Silty Lake Pesaquid 1970 Fresh water Impacts on the Windsor Salt Marsh (Ramsar Wetland & IBA of Canada) Unlike the Petitcodiac – keeping an aboiteau EA completed in 2017 – currently working on design Project in planning for almost 20 years – including various environmental studies of the Avon River Estuary Contracted Acadia University, St. Mary’s University and CBWES Inc., between 2002 and 2018 to better understand the estuary and inform our design team to minimize impacts on salt marsh and mudflats. Baseline CRA Fisheries Study (Commercial, Recreational and Aboriginal) Contracted 3 partners for work between April 2017 and March 2019 ➢ Darren Porter, commercial fisher, ➢ Acadia University (Dr. Trevor Avery) ➢ Mi’kmaq Conservation Group Key study goal to better inform the detailed design team to improve fish passage through the aboiteau (sluice) Just before Christmas 2017, we engaged a team led by CBCL Limited to design an upgraded causeway and aboiteau system. Design Objectives Public Safety • Maintain corridor over Avon River for Highway 101 Twinning and continuity of rail, trail and utility services. • Continued protection of communities and agricultural land from the effects of flooding and sea level rise / climate change. Regulatory Requirements • Improve fish passage (EA Condition & Fisheries Act ). • Minimize environmental impacts (i.e., impact to salt marsh). • Consideration of potential negative impacts to asserted or established Mi’kmaq aboriginal or treaty rights. -

Support for Delineation of Inner Bay of Fundy Salmon Marine Critical Habitat Boundaries in Minas Basin and Chignecto
Canadian Science Advisory Secretariat Maritimes Region Science Response 2015/035 SUPPORT FOR DELINEATION OF INNER BAY OF FUNDY SALMON MARINE CRITICAL HABITAT BOUNDARIES IN MINAS BASIN AND CHIGNECTO BAY Context In April 2014, the Fisheries and Oceans Canada (DFO) Species at Risk Management Division (SARMD) in the Maritimes Region requested information from DFO Science to assist with the delineation of boundaries for critical habitat (CH) being considered for Inner Bay of Fundy (IBOF) Atlantic Salmon within Chignecto Bay and Minas Basin, specifically: to assist with the delineation of the boundary between estuarine and marine habitat for several large, tidal estuaries (i.e., Petitcodiac River, Avon River, Salmon River Colchester, Shubenacadie River estuary and Cumberland Basin). DFO Science had previously provided advice on the characteristics and general location of important marine and estuarine habitat for IBOF salmon (DFO 2008; DFO 2013); however, additional information was requested to assist in delineating the precise boundaries of important marine habitat within Chignecto Bay and Minas Basin in order to subsequently propose, describe and map these as CH within an amended Recovery Strategy for IBOF salmon. Once identified in the Recovery Strategy, measures will be taken to protect this marine CH under the Species at Risk Act (SARA). This Science Response Report results from the Science Response Process of 11 July 2014 on Support for Delineation of Inner Bay of Fundy Salmon Marine Critical Habitat Boundaries. Background The inner Bay of Fundy populations of Atlantic salmon (Salmo salar) are listed as Endangered under the Species at Risk Act, and SARA requires the identification of CH for endangered species within a Recovery Strategy (or Action Plan). -

Environmental Implications of Expanding the Windsor Causeway (Part 2)
Environmental Implications of Expanding the Windsor Causeway (Part 2): Comparison of 4 and 6 Lane Options Final Report prepared for Nova Scotia Department of Transportation and Public Works ACER Report No. 75 2 Environmental Implications of Expanding the Windsor Causeway (Part 2): Comparison of 4 and 6 Lane Options Report Prepared for Nova Scotia Department of Transportation and Public Works. Contract # 02-00026 Prepared by Danika van Proosdij, Graham R. Daborn and Michael Brylinsky April 2004. 3 1.0 Introduction Preliminary plans for twinning Highway 101 include expansion of the width of the Windsor Causeway to accommodate two or four additional lanes, creating a four or six lane divided highway. Because of the limitations imposed by infrastructure in the Town of Windsor, and by Fort Edward, such expansion is feasible only on the seaward side of the existing structure. Realignment of the existing roadway would also be designed to decrease the sharp curve at the western end, which currently requires a speed limit of 90 km per hour. The new construction would therefore cover part of the marsh and tidal channel adjacent to the existing causeway. During 2002, studies of the mudflat—saltmarsh complex on the seaward side of the Windsor Causeway and of Pesaquid Lake, were carried out, in part, to provide information relevant to an assessment of the ecological implications of such an expansion. These studies also constituted the first step in a planned long term monitoring of continuing evolution of the marsh-mudflat complex that has resulted from construction of the original causeway. Reports on the 2002 studies were presented as ACER Publications 69 and 72 in 2003 (Daborn et al. -

Ecosystem Overview Report for the Minas Basin, Nova Scotia
Ecosystem Overview Report for the Minas Basin, Nova Scotia Prepared for: Oceans and Habitat Branch Maritimes Region Fisheries and Oceans Canada Bedford Institute of Oceanography PO Box 1006 Dartmouth, Nova Scotia B2Y 4A2 Prepared by: M. Parker1, M. Westhead2 and A. Service1 1East Coast Aquatics Inc. Bridgetown, Nova Scotia 2Fisheries and Oceans Canada, Bedford Institute of Oceans and Coastal Management Report 2007-05 Oceans and Habitat Report Series The Oceans and Habitat Report Series contains public discussion papers, consultant reports, and other public documents prepared for and by the Oceans and Habitat Branch, Fisheries and Oceans Canada, Maritimes Region. Documents in the series reflect the broad interests, policies and programs of Fisheries and Oceans Canada. The primary focus of the series is on topics related to oceans and coastal planning and management, conservation, habitat protection and sustainable development. Documents in the series are numbered chronologically by year of publication. The series commenced with Oceans and Coastal Management Report No. 1998-01. In 2007, the name was changed to the Oceans and Habitat Report Series. Documents are available through the Oceans and Habitat Branch in both electronic and limited paper formats. Reports of broad international, national, regional or scientific interest may be catalogued jointly with other departmental document series, such as the Canadian Technical Report of Fisheries and Aquatic Sciences Series. Série des Rapports sur l’habitat et les océans La série des Rapports sur l’habitat et les océans regroupe des documents de discussion publics, des rapports d’experts et d’autres documents publics préparés par la Direction des océans et de l’habitat de Pêches et Océans Canada, Région des Maritimes ou pour le compte de cette direction. -

Working with Minas Basin Watershed Community Groups
WORKING WITH MINAS BASIN WATERSHED COMMUNITY GROUPS SUMMARY REPORT Prepared by Lisa M. McCuaig of the Minas Basin Working Group Bay of Fundy Ecosystem Partnership FEBRUARY 2004 TABLE OF CONTENTS Page # Acknowledgements………………………………………………………4 1. Executive Summary …………………………………………………..5 1.1 Achievements (tangible)………………………………………………………..5 1.2 Achievements (immeasurable)………………………………………………...6 2. Introduction……………………………………………………………..7 2.1 Background………………………………………………………………………7 2.2 Mission and Objectives of the Minas Basin Working Group………………..7 2.3 Challenges………………………………………………………………….…….8 2.4 Recommendations……………………………………………………………...10 2.5 Successful Outcomes…………………………………………………………..11 2.6 Overall Status of Groups……………………………………………………….12 3. Discussion on Action Planning……………………………………..13 3.1 Introduction………………………………………………………………………13 3.2 Successful Outcomes…………………………………………………………..14 3.3 Challenges / Areas for Improvement………………………………………….14 3.4 Next Steps……………………………………………………………………….15 3.5 Future Groups to Work on Action Plans……………………………………...15 3.6 Groups Not Requiring MBWG Assistance……………………………………15 3.7 Completed Action Plans………………………………………………………..16 4. Workshop / Meeting Organisation………………………………….17 4.1 Introduction………………………………………………………………………17 4.2 Successful Outcomes…………………………………………………………..17 4.3 Challenges……………………………………………………………………….17 4.4 Areas for Improvement / Next Steps………………………………………….17 4.5 Summary of Workshops………………………………………………………..18 4.6 Summary of Meetings…………………………………………………………..18 4.7 Future Workshops and Meetings………………………………………………18 -

Halifax 2005
GAC-MAC-CSPG-CSSS Pre-conference Field Trips A1 Contamination in the South Mountain Batholith and Port Mouton Pluton, southern Nova Scotia HALIFAX Building Bridges—across science, through time, around2005 the world D. Barrie Clarke and Saskia Erdmann A2 Salt tectonics and sedimentation in western Cape Breton Island, Nova Scotia Ian Davison and Chris Jauer A3 Glaciation and landscapes of the Halifax region, Nova Scotia Ralph Stea and John Gosse A4 Structural geology and vein arrays of lode gold deposits, Meguma terrane, Nova Scotia Rick Horne A5 Facies heterogeneity in lacustrine basins: the transtensional Moncton Basin (Mississippian) and extensional Fundy Basin (Triassic-Jurassic), New Brunswick and Nova Scotia David Keighley and David E. Brown A6 Geological setting of intrusion-related gold mineralization in southwestern New Brunswick Kathleen Thorne, Malcolm McLeod, Les Fyffe, and David Lentz A7 The Triassic-Jurassic faunal and floral transition in the Fundy Basin, Nova Scotia Paul Olsen, Jessica Whiteside, and Tim Fedak Post-conference Field Trips B1 Accretion of peri-Gondwanan terranes, northern mainland Nova Scotia Field Trip B6 and southern New Brunswick Sandra Barr, Susan Johnson, Brendan Murphy, Georgia Pe-Piper, David Piper, and Chris White The macrotidal environment of the Minas Basin, B2 The Joggins Cliffs of Nova Scotia: Lyell & Co's "Coal Age Galapagos" J.H. Calder, M.R. Gibling, and M.C. Rygel Nova Scotia: sedimentology,morphology, B3 Geology and volcanology of the Jurassic North Mountain Basalt, southern Nova Scotia Dan -

Recovery Potential Assessment for Inner Bay of Fundy Atlantic Salmon
Canadian Science Advisory Secretariat Maritimes Region Science Advisory Report 2008/050 RECOVERY POTENTIAL ASSESSMENT FOR INNER BAY OF FUNDY ATLANTIC SALMON Figure 1. Map showing the region within the Maritimes Provinces where inner Bay of Fundy Atlantic salmon are found. Context: Inner Bay of Fundy (iBoF) Atlantic salmon were listed as endangered under Schedule 1 of the Species at Risk Act (SARA) when it came into force in 2004, and they obtained protection (illegal to kill, harm, harass, capture, take, etc.) under SARA at this time. A recovery team for iBoF salmon was in existence and was very active prior to the enactment of SARA, and a Recovery Strategy was developed for this species prior to SARA coming into force. However, SARA has specific elements that must be included in a Recovery Strategy, and a revised draft has been under development since 2003. In advance of finalizing the Strategy, DFO Science has been asked to undertake a Recovery Potential Assessment (RPA) based on the National Protocol to inform the scientific elements of the Recovery Strategy. The advice generated via this process will also update and consolidate existing advice on iBoF salmon. This report provides a summary of current understanding related to the distribution, abundance, trends, extinction risk and current state of iBoF salmon populations. Information on habitat requirements as well as threats to both habitat and salmon are also included. Proposed recovery targets are described, and results of population modeling help to better understand the likelihood of achieving these targets under various scenarios. SUMMARY Wild iBoF salmon have declined to critically low levels and are currently at risk of extinction. -

2020-10-01 Windsor Causeway Survey Report
Windsor Causeway Survey Report September 2020 Table of Contents Methodology & Logistics 2 Awareness of Fish Passage Restriction 3 Restoring Free Passage of Fish – Support 4 Bridge – Support 4 Sipekne’katik First Nation Request 5 Highway Construction Plans 5 New Structure Design 6 Motivators 7 Lake Pisiquid 8 Results by Question 9 1 Background & Overview The following report presents the research findings from a survey conducted by Oraclepoll Research Ltd for Oceans North and the Mi’kmaw Conservation Group. It involved collecting telephone research among residents 18 years of age and older from Windsor Hants County, Nova Scotia on issues related to the Windsor Causeway. Methodology & Logistics Study Sample & Survey Method A total of N=300 interviews were completed among residents 18 years of age and older between the days of August 30th to September 5th, 2020. This survey was conducted by telephone with live operators using computer-assisted telephone interviewing (CATI) and random digit dialing number selection (RDD). A dual-frame sample database was used that contained cellular and landline phone number contacts. No financial incentives were used, and respondents were assured of confidentiality and that the information they provided was for research purposes only. Oraclepoll adheres to strict privacy codes and no personal identifiers (in this case telephone numbers) will be shared with any outside party or will be reported. Logistics The data collection period was from August 30th to September 5th, 2020. Initial calls were made between the hours of 6:00 p.m. and 9:00 p.m. Subsequent call-backs of no- answers and busy numbers were made up to 5 times over the call period (from 10:00 a.m.