What Drives Neutrophils to the Alveoli in ARDS?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recapitulates Some Features of Psoriasis Α , And
The Journal of Immunology Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1a, and TNF-a Recapitulates Some Features of Psoriasis Karline Guilloteau,*,† Isabelle Paris,*,‡ Nathalie Pedretti,*,† Katia Boniface,*,1 Franck Juchaux,*,† Vincent Huguier,x Gerard Guillet,{ Franc¸ois-Xavier Bernard,*,† Jean-Claude Lecron,*,‡ and Franck Morel* Keratinocytes play a crucial role in the regulation of skin inflammation, responding to environmental and immune cells stimuli. They produce soluble factors that can act in an autocrine or paracrine manner on immune cells or directly on aggressors. A screen- ing of the activities of 36 cytokines on keratinocyte gene expression identified IL-17A, IL-22, oncostatin M, TNF-a, and IL-1a as potent cytokines in inducing cutaneous inflammation. These five proinflammatory cytokines synergistically increased production of CXCL8 and b-defensin 2 (BD2). In addition, ex vivo studies on human skin explants demonstrated upregulation of BD2, S100A7, and CXCL8 expression in response to the same combination of cytokines. In vivo intradermal injection of these five cytokines in mouse increased CXCL1, CXCL2, CXCL3, S100A9, and BD3 expression, associated with neutrophil infiltration. We confirmed and extended this synergistic effect using quantitative real-time PCR analysis and observed increased expression of nine chemokines and 12 antimicrobial peptides. Production of CXCL, CXCL5, and CXCL8 by keratinocytes stimulated in the presence of this cytokine combination was associated with increased neutrophil chemotactic activity. Similarly, high production of BD2, BD3, and S100A7 was associated with an increased antimicrobial activity. Finally, the transcriptional profile observed in this in vitro model of inflammatory keratinocytes correlated with the one of lesional psoriatic skin. -

Inhibition of CCL7 Derived from Mo-Mdscs Prevents Metastatic
Ren et al. Cell Death and Disease (2021) 12:484 https://doi.org/10.1038/s41419-021-03698-5 Cell Death & Disease ARTICLE Open Access Inhibition of CCL7 derived from Mo-MDSCs prevents metastatic progression from latency in colorectal cancer Xiaoli Ren1,2, Jianbiao Xiao1,3, Wanning Zhang1,3,FeifeiWang1,3, Yongrong Yan1,3,XuehuiWu 1,3, Zhicheng Zeng1,3, Yumei He4,WeiYang1,3, Wangjun Liao5,YanqingDing1,3 and Li Liang 1,3 Abstract In colorectal cancer (CRC), overt metastases often appear after years of latency. But the signals that cause micro- metastatic cells to remain indolent, thereby enabling them to survive for extended periods of time, are unclear. Immunofluorescence and co-immunoprecipitation assays were used to explore the co-localization of CCL7 and CCR2. Immunohistochemical (IHC) assays were employed to detect the characters of metastatic HT29 cells in mice liver. Flow cytometry assays were performed to detect the immune cells. Bruberin vivo MS FX Pro Imager was used to observe the liver metastasis of CRC in mice. Quantitative real-time PCR (qRT-PCR) and western blot were employed to detect the expressions of related proteins. Trace RNA sequencing was employed to identify differentially expressed genes in MDSCs from liver micro-M and macro-M of CRC in mice. Here, we firstly constructed the vitro dormant cell models and metastatic dormant animal models of colorectal cancer. Then we found that myeloid-derived suppressor cells (MDSCs) were increased significantly from liver micro-metastases to macro-metastases of CRC in mice. Moreover, monocytic MDSCs (Mo-MDSC) significantly promoted the dormant activation of micro-metastatic cells compared to 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; polymorphonuclear MDSCs (PMN-MDSC). -

CCL5 Secreted by Senescent Aged Fibroblasts Induces Proliferation of Prostate Epithelial Cells and Expression of Genes That Modu
JCP-09-0003.R1(21776) ORIGINAL ARTICLE 1 JJ o o u u r r n n a a l l oo f f Cellular CCL5 Secreted by Senescent Aged Physiology Fibroblasts Induces Proliferation of Prostate Epithelial Cells and Expression of Genes that Modulate AngiogenesisQ1 1 1 2 1 D. EYMAN, M. DAMODARASAMY, S.R. PLYMATE, AND M.J. REED * 1Department of Medicine, Harborview Medical Center, University of Washington, Seattle, Washington 2Veterans Affairs Puget Sound Health Care System, Seattle Washington There is increased interest in the effects of secretory products from aged cells on promoting both benign and malignant cell growth. We identified a human fibroblast line, AG04382, from an aged donor that naturally demonstrated senescence-associated features and whose conditioned media significantly induced proliferation of benign prostatic hyperplasia (BPH1) cells. Candidate cytokines mediating this effect were identified with protein arrays and validated by ELISA. We found that the AG04382 fibroblast line secreted high levels of CXCL5, CCL5, and CCL2, but relative to the other lines, its conditioned media was unique in its increased expression of CCL5. Blocking studies using specific antibodies against CXCL5, CCL5, and CCL2 in the conditioned media of AG04382 showed that only CCL5 contributed significantly to BPH1 proliferation. Stimulation of BPH1 cells with rhuCCL5 resulted in increased proliferation and migration, as well as significant changes in the expression of genes that influence angiogenesis. These data suggest that CCL5 is a candidate chemokine secreted by aged cells that promotes prostate growth and regulates angiogenesis. J. Cell. Physiol. 9999: 1–6, 2009. ß 2009 Wiley-Liss, Inc. There is a close correlation between host age and the analyses for potential paracrine modulators demonstrated that prevalence of both benign (prostatic hyperplasia) and malignant the chemokine, CCL5, in the conditioned media of the aged (cancer) prostate disease (Balducci and Ershler, 2005; fibroblasts was responsible, in part, for inducing proliferation of Untergasser et al., 2005; Nelen, 2007). -

The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature
International Journal of Molecular Sciences Review The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature Jan Korbecki 1 , Klaudyna Kojder 2, Patrycja Kapczuk 1, Patrycja Kupnicka 1 , Barbara Gawro ´nska-Szklarz 3 , Izabela Gutowska 4 , Dariusz Chlubek 1 and Irena Baranowska-Bosiacka 1,* 1 Department of Biochemistry and Medical Chemistry, Pomeranian Medical University in Szczecin, Powsta´nców Wielkopolskich 72 Av., 70-111 Szczecin, Poland; [email protected] (J.K.); [email protected] (P.K.); [email protected] (P.K.); [email protected] (D.C.) 2 Department of Anaesthesiology and Intensive Care, Pomeranian Medical University in Szczecin, Unii Lubelskiej 1, 71-281 Szczecin, Poland; [email protected] 3 Department of Pharmacokinetics and Therapeutic Drug Monitoring, Pomeranian Medical University in Szczecin, Powsta´nców Wielkopolskich 72 Av., 70-111 Szczecin, Poland; [email protected] 4 Department of Medical Chemistry, Pomeranian Medical University in Szczecin, Powsta´nców Wlkp. 72 Av., 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-914661515 Abstract: Hypoxia is an integral component of the tumor microenvironment. Either as chronic or cycling hypoxia, it exerts a similar effect on cancer processes by activating hypoxia-inducible factor-1 (HIF-1) and nuclear factor (NF-κB), with cycling hypoxia showing a stronger proinflammatory influ- ence. One of the systems affected by hypoxia is the CXC chemokine system. This paper reviews all available information on hypoxia-induced changes in the expression of all CXC chemokines (CXCL1, CXCL2, CXCL3, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8 (IL-8), CXCL9, CXCL10, CXCL11, CXCL12 Citation: Korbecki, J.; Kojder, K.; Kapczuk, P.; Kupnicka, P.; (SDF-1), CXCL13, CXCL14, CXCL15, CXCL16, CXCL17) as well as CXC chemokine receptors— Gawro´nska-Szklarz,B.; Gutowska, I.; CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CXCR7 and CXCR8. -

Mouse CXCL15 ELISA Kit (ARG82062)
Product datasheet [email protected] ARG82062 Package: 96 wells Mouse CXCL15 ELISA Kit Store at: 4°C Summary Product Description Mouse CXCL15 ELISA Kit is an Enzyme Immunoassay kit for the quantification of Mouse CXCL15 in serum, plasma and cell culture supernatants. Tested Reactivity Ms Tested Application ELISA Target Name CXCL15 Conjugation HRP Conjugation Note Substrate: TMB and read at 450 nm. Sensitivity 62.5 pg/ml Sample Type Serum, plasma and cell culture supernatants. Standard Range 125 - 8000 pg/ml Sample Volume 100 µl Alternate Names Scyb15; weche; Small-inducible cytokine B15; C-X-C motif chemokine 15; Lungkine; lungkine Application Instructions Assay Time 4.5 hours Properties Form 96 well Storage instruction Store the kit at 2-8°C. Keep microplate wells sealed in a dry bag with desiccants. Do not expose test reagents to heat, sun or strong light during storage and usage. Please refer to the product user manual for detail temperatures of the components. Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Gene Symbol Cxcl15 Gene Full Name chemokine (C-X-C motif) ligand 15 Function Chemotactic for neutrophils. Involved in lung-specific neutrophil trafficking during normal and inflammatory conditions. [UniProt] Highlight Related products: CXCL antibodies; CXCL ELISA Kits; CXCL Duos / Panels; CXCL recombinant proteins; New ELISA data calculation tool: Simplify the ELISA analysis by GainData www.arigobio.com 1/2 Images ARG82062 Mouse CXCL15 ELISA Kit standard curve image ARG82062 Mouse CXCL15 ELISA Kit results of a typical standard run with optical density reading at 450 nm. www.arigobio.com 2/2 Powered by TCPDF (www.tcpdf.org). -

The Chemokine System in Innate Immunity
Downloaded from http://cshperspectives.cshlp.org/ on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press The Chemokine System in Innate Immunity Caroline L. Sokol and Andrew D. Luster Center for Immunology & Inflammatory Diseases, Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 02114 Correspondence: [email protected] Chemokines are chemotactic cytokines that control the migration and positioning of immune cells in tissues and are critical for the function of the innate immune system. Chemokines control the release of innate immune cells from the bone marrow during homeostasis as well as in response to infection and inflammation. Theyalso recruit innate immune effectors out of the circulation and into the tissue where, in collaboration with other chemoattractants, they guide these cells to the very sites of tissue injury. Chemokine function is also critical for the positioning of innate immune sentinels in peripheral tissue and then, following innate immune activation, guiding these activated cells to the draining lymph node to initiate and imprint an adaptive immune response. In this review, we will highlight recent advances in understanding how chemokine function regulates the movement and positioning of innate immune cells at homeostasis and in response to acute inflammation, and then we will review how chemokine-mediated innate immune cell trafficking plays an essential role in linking the innate and adaptive immune responses. hemokines are chemotactic cytokines that with emphasis placed on its role in the innate Ccontrol cell migration and cell positioning immune system. throughout development, homeostasis, and in- flammation. The immune system, which is de- pendent on the coordinated migration of cells, CHEMOKINES AND CHEMOKINE RECEPTORS is particularly dependent on chemokines for its function. -

Predictive Biomarkers for the Ranking of Pulmonary Toxicity of Nanomaterials
nanomaterials Article Predictive Biomarkers for the Ranking of Pulmonary Toxicity of Nanomaterials Chinatsu Nishida 1, Hiroto Izumi 2, Taisuke Tomonaga 2 , Jun-ichi Takeshita 3 , Ke-Yong Wang 4, Kei Yamasaki 1, Kazuhiro Yatera 1 and Yasuo Morimoto 2,* 1 Department of Respiratory Medicine, University of Occupational and Environmental Health, Japan. 1-1 Iseigaoka, Yahata-nishi-ku, Kitakyushu, Fukuoka 807-8555, Japan; [email protected] (C.N.); [email protected] (K.Y.); [email protected] (K.Y.) 2 Department of Occupational Pneumology, Institute of Industrial Ecological Sciences, University of Occupational and Environmental Health, Japan. 1-1 Iseigaoka, Yahata-nishi-ku, Kitakyushu, Fukuoka 807-8555, Japan; [email protected] (H.I.); [email protected] (T.T.) 3 Research Institute of Science for Safety and Sustainability, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan. 16-1 Onogawa, Tsukuba, Ibaraki 305-8569, Japan; [email protected] 4 Shared-Use Research Center, University of Occupational and Environmental Health, Japan. 1-1 Iseigaoka, Yahata-nishi-ku, Kitakyushu, Fukuoka 807-8555, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-93-691-7136 Received: 3 September 2020; Accepted: 9 October 2020; Published: 15 October 2020 Abstract: We analyzed the mRNA expression of chemokines in rat lungs following intratracheal instillation of nanomaterials in order to find useful predictive markers of the pulmonary toxicity of nanomaterials. Nickel oxide (NiO) and cerium dioxide (CeO2) as nanomaterials with high pulmonary toxicity, and titanium dioxide (TiO2) and zinc oxide (ZnO) as nanomaterials with low pulmonary toxicity, were administered into rat lungs (0.8 or 4 mg/kg BW). -

Chloride Channels Regulate Differentiation and Barrier Functions
RESEARCH ARTICLE Chloride channels regulate differentiation and barrier functions of the mammalian airway Mu He1†*, Bing Wu2†, Wenlei Ye1, Daniel D Le2, Adriane W Sinclair3,4, Valeria Padovano5, Yuzhang Chen6, Ke-Xin Li1, Rene Sit2, Michelle Tan2, Michael J Caplan5, Norma Neff2, Yuh Nung Jan1,7,8, Spyros Darmanis2*, Lily Yeh Jan1,7,8* 1Department of Physiology, University of California, San Francisco, San Francisco, United States; 2Chan Zuckerberg Biohub, San Francisco, United States; 3Department of Urology, University of California, San Francisco, San Francisco, United States; 4Division of Pediatric Urology, University of California, San Francisco, Benioff Children’s Hospital, San Francisco, United States; 5Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Heaven, United States; 6Department of Anesthesia and Perioperative Care, University of California, San Francisco, San Francisco, United States; 7Department of Biochemistry and Biophysics, University of California, San Francisco, San Francisco, United States; 8Howard Hughes Medical Institute, University of California, San Francisco, San Francisco, United States *For correspondence: Abstract The conducting airway forms a protective mucosal barrier and is the primary target of [email protected] (MH); [email protected] airway disorders. The molecular events required for the formation and function of the airway (SD); mucosal barrier, as well as the mechanisms by which barrier dysfunction leads to early onset airway [email protected] (LYJ) diseases, -

CXCL5 Signaling Is a Shared Pathway of Neuroinflammation and Blood
Wang et al. Journal of Neuroinflammation (2016) 13:6 DOI 10.1186/s12974-015-0474-6 RESEARCH Open Access CXCL5 signaling is a shared pathway of neuroinflammation and blood–brain barrier injury contributing to white matter injury in the immature brain Lin-Yu Wang1,2, Yi-Fang Tu3,4, Yung-Chieh Lin3,4 and Chao-Ching Huang3,5,6* Abstract Background: In very preterm infants, white matter injury is a prominent brain injury, and hypoxic ischemia (HI) and infection are the two primary pathogenic factors of this injury. Microglia and microvascular endothelial cells closely interact; therefore, a common signaling pathway may cause neuroinflammation and blood–brain barrier (BBB) damage after injury to the immature brain. CXC chemokine ligand 5 (CXCL5) is produced in inflammatory and endothelial cells by various organs in response to insults. CXCL5 levels markedly increased in the amniotic cavity in response to intrauterine infection and preterm birth in clinical studies. The objective of this study is to determine whether CXCL5 signaling is a shared pathway of neuroinflammation and BBB injury that contributes to white matter injury in the immature brain. Methods: Postpartum day 2 (P2) rat pups received lipopolysaccharide (LPS) followed by 90-min HI. Immunohistochemical analyses were performed to determine microglial activation, neutrophil infiltration, BBB damage, and myelin basic protein and glial fibrillary acidic protein expression. Immunofluorescence experiments were performed to determine the cellular distribution of CXCL5. Pharmacological tests were performed to inhibit or enhance CXCL5 activity. Results: On P2, predominant increases in microglial activation and BBB damage were observed 24 h after LPS-sensitized HI induction, and white matter injury (decreasedmyelinationandincreasedastrogliosis) was observed on P12 compared with controls. -

The CXCL5/CXCR2 Axis Is Sufficient to Promote Breast Cancer Colonization During Bone Metastasis
ARTICLE https://doi.org/10.1038/s41467-019-12108-6 OPEN The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis Ricardo Romero-Moreno1,2, Kimberly J. Curtis1,3, Thomas R. Coughlin1,3, Maria Cristina Miranda-Vergara1,2, Shourik Dutta1,2, Aishwarya Natarajan1,2, Beth A. Facchine1,2, Kristen M. Jackson1,3, Lukas Nystrom5,6, Jun Li1,4, William Kaliney1, Glen L. Niebur 1,3 & Laurie E. Littlepage 1,2 Bone is one of the most common sites for metastasis across cancers. Cancer cells that travel 1234567890():,; through the vasculature and invade new tissues can remain in a non-proliferative dormant state for years before colonizing the metastatic site. Switching from dormancy to colonization is the rate-limiting step of bone metastasis. Here we develop an ex vivo co-culture method to grow cancer cells in mouse bones to assess cancer cell proliferation using healthy or cancer- primed bones. Profiling soluble factors from conditioned media identifies the chemokine CXCL5 as a candidate to induce metastatic colonization. Additional studies using CXCL5 recombinant protein suggest that CXCL5 is sufficient to promote breast cancer cell pro- liferation and colonization in bone, while inhibition of its receptor CXCR2 with an antagonist blocks proliferation of metastatic cancer cells. This study suggests that CXCL5 and CXCR2 inhibitors may have efficacy in treating metastatic bone tumors dependent on the CXCL5/ CXCR2 axis. 1 Harper Cancer Research Institute, South Bend, IN, USA. 2 Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN, USA. 3 Tissue Mechanics Laboratory, Department of Aerospace and Mechanical Engineering, Bioengineering Graduate Program, University of Notre Dame, Notre Dame, IN, USA. -
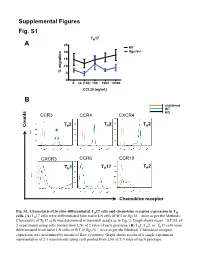
Supplemental Figures 1-3 and Supplemental Table 1 (PDF, 155
Supplemental Figures Fig. S1 TH17 A 25 WT 20 Rgs16-/- 15 10 % migration 5 0 0. ck (100) 100 1000 10000 CCL20 (ng/mL) B unstained WT KO CCR3 CCR4 CXCR4 T 2 T 2 T 2 Counts H H H CXCR3 CCR6 CCR10 T 2 TH1 TH17 H Chemokine receptor in vitro- Fig. S1. Chemotaxis of differentiated TH17 cells and chemokine receptor expression in TH –/– cells. (A) TH17 cells were differentiated from naïve LN cells of WT or Rgs16 mice as per the Methods. Chemotaxis of TH17 cells was determined in transwell assays as in Fig. 2. Graph shows mean ± S.E.M. of 2 experiments using cells pooled from LNs of 2 mice of each genotype. (B) TH1, TH2, or TH17 cells were differentiated from naïve LN cells of WT or Rgs16–/– mice as per the Methods. Chemokine receptor expression was determined by means of flow cytometry. Graph shows results of a single experiment representative of 2-3 experiments using cells pooled from LNs of 2-3 mice of each genotype. Fig. S2 A B 2.5 ) 6 2.0 1.5 1.0 Cells (x 10 0.5 0.0 WT Rgs16-/- Fig. S2. Intact proliferation and development RGS16-deficient TH cells. –/– (A) TH2 cells were differentiated from naïve LN cells of WT or Rgs16 mice as per the Methods. Proliferation of untreated (middle) or anti-CD3/ anti-CD28-treated (right) cells was determined by means of CFSE labeling –/– and flow cytometry. (B) Differentiated TH2 cells WT or Rgs16 mice were quantified after a 3 day incubation in basal medium. -

Targeting Tumor Cell-Derived CCL2 As a Strategy to Overcome Bevacizumab Resistance in ETV5+ Colorectal Cancer
Feng et al. Cell Death and Disease (2020) 11:916 https://doi.org/10.1038/s41419-020-03111-7 Cell Death & Disease ARTICLE Open Access Targeting tumor cell-derived CCL2 as a strategy to overcome Bevacizumab resistance in ETV5+ colorectal cancer Haoran Feng1,2,KunLiu1,3,XiaonanShen4,JuyongLiang1,2, Changgang Wang1,3,WeihuaQiu1,3,2,XiCheng 1,3,2 and Ren Zhao1,3,2 Abstract In our previous study, ETV5 mediated-angiogenesis was demonstrated to be dependent upon the PDGF-BB/PDGFR-β/ Src/STAT3/VEGFA pathway in colorectal cancer (CRC). However, the ability of ETV5 to affect the efficacy of anti- angiogenic therapy in CRC requires further investigation. Gene set enrichment analysis (GSEA) and a series of experiments were performed to identify the critical candidate gene involved in Bevacizumab resistance. Furthermore, the ability of treatment targeting the candidate gene to enhance Bevacizumab sensitivity in vitro and in vivo was investigated. Our results revealed that ETV5 directly bound to the VEGFA promoter to promote translation of VEGFA. However, according to in vitro and in vivo experiments, ETV5 unexpectedly accelerated antiVEGF therapy (Bevacizumab) resistance. GSEA and additional assays confirmed that ETV5 could promote angiogenesis by inducing the secretion of another tumor angiogenesis factor (CCL2) in CRC cells to facilitate Bevacizumab resistance. Mechanistically, ETV5 upregulated CCL2 by activating STAT3 to facilitate binding with the CCL2 promoter. ETV5 induced-VEGFA translation and CCL2 secretion were mutually independent mechanisms, that induced angiogenesis 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; by activating the PI3K/AKT and p38/MAPK signaling pathways in human umbilical vein endothelial cells (HUVECs). In CRC tissues, ETV5 protein levels were positively associated with CD31, CCL2, and VEGFA protein expression.