Fallen in a Dead Ear: Intralabyrinthine Preservation of Stapes in Fossil Artiodactyls Maeva Orliac, Guillaume Billet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JVP 26(3) September 2006—ABSTRACTS
Neoceti Symposium, Saturday 8:45 acid-prepared osteolepiforms Medoevia and Gogonasus has offered strong support for BODY SIZE AND CRYPTIC TROPHIC SEPARATION OF GENERALIZED Jarvik’s interpretation, but Eusthenopteron itself has not been reexamined in detail. PIERCE-FEEDING CETACEANS: THE ROLE OF FEEDING DIVERSITY DUR- Uncertainty has persisted about the relationship between the large endoskeletal “fenestra ING THE RISE OF THE NEOCETI endochoanalis” and the apparently much smaller choana, and about the occlusion of upper ADAM, Peter, Univ. of California, Los Angeles, Los Angeles, CA; JETT, Kristin, Univ. of and lower jaw fangs relative to the choana. California, Davis, Davis, CA; OLSON, Joshua, Univ. of California, Los Angeles, Los A CT scan investigation of a large skull of Eusthenopteron, carried out in collaboration Angeles, CA with University of Texas and Parc de Miguasha, offers an opportunity to image and digital- Marine mammals with homodont dentition and relatively little specialization of the feeding ly “dissect” a complete three-dimensional snout region. We find that a choana is indeed apparatus are often categorized as generalist eaters of squid and fish. However, analyses of present, somewhat narrower but otherwise similar to that described by Jarvik. It does not many modern ecosystems reveal the importance of body size in determining trophic parti- receive the anterior coronoid fang, which bites mesial to the edge of the dermopalatine and tioning and diversity among predators. We established relationships between body sizes of is received by a pit in that bone. The fenestra endochoanalis is partly floored by the vomer extant cetaceans and their prey in order to infer prey size and potential trophic separation of and the dermopalatine, restricting the choana to the lateral part of the fenestra. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

NM IF C3 4 16 Fossil Imprint
FOSSIL IMPRINT • vol. 72 • 2016 • no. 3-4 • pp. 183–201 (formerly ACTA MUSEI NATIONALIS PRAGAE, Series B – Historia Naturalis) HIPPOPOTAMODON ERYMANTHIUS (SUIDAE, MAMMALIA) FROM MAHMUTGAZI, DENIZLI-ÇAL BASIN, TURKEY MARTIN PICKFORD Sorbonne Universités – CR2P, MNHN, CNRS, UPMC – Paris VI, 8, rue Buffon, 75005, Paris, France; e-mail: [email protected]. Pickford, M. (2016): Hippopotamodon erymanthius (Suidae, Mammalia) from Mahmutgazi, Denizli-Çal Basin, Turkey. – Fossil Imprint, 72(3-4): 183–201, Praha. ISSN 2533-4050 (print), ISSN 2533-4069 (on-line). Abstract: The Staatliches Museum für Naturkunde in Karlsruhe houses an interesting collection of Turolian mammals from Mahmutgazi, Turkey, among which is a comprehensive sample of the large suid, Hippopotamodon erymanthius. The fossils plot out within the range of metric variation of H. erymanthius from Pikermi and Samos, Greece, but lie at the lower end of the range. Like the suids from these sites, the Mahmutgazi specimens lack the first premolar. Overall, the Mahmutgazi sample is metrically and morphologically close to the material from Akkaşdağı, Turkey. The upper and lower third molars and fourth premolars are, on average, smaller than those of Hippopotamodon major from Luberon, France (MN 13). Two undescribed fossils of H. ery- manthius from Pikermi are housed at the SMNK, and are included in this paper in order to fill out the data base for the species at this locality. The chronological position, palaeoecology and sexual dimorphism of the Mahmutgazi suids are discussed. Key words: Suidae, Late Miocene, Turkey, Hippopotamodon, biochronology, palaeoecology, sexual dimorphism Received: October 10, 2016 | Accepted: November 28, 2016 | Issued: December 30, 2016 Introduction (2010) mentions suids at the site, and correlated it to MN 11–12 (Early to Middle Turolian). -

Chapter 1 - Introduction
EURASIAN MIDDLE AND LATE MIOCENE HOMINOID PALEOBIOGEOGRAPHY AND THE GEOGRAPHIC ORIGINS OF THE HOMININAE by Mariam C. Nargolwalla A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Anthropology University of Toronto © Copyright by M. Nargolwalla (2009) Eurasian Middle and Late Miocene Hominoid Paleobiogeography and the Geographic Origins of the Homininae Mariam C. Nargolwalla Doctor of Philosophy Department of Anthropology University of Toronto 2009 Abstract The origin and diversification of great apes and humans is among the most researched and debated series of events in the evolutionary history of the Primates. A fundamental part of understanding these events involves reconstructing paleoenvironmental and paleogeographic patterns in the Eurasian Miocene; a time period and geographic expanse rich in evidence of lineage origins and dispersals of numerous mammalian lineages, including apes. Traditionally, the geographic origin of the African ape and human lineage is considered to have occurred in Africa, however, an alternative hypothesis favouring a Eurasian origin has been proposed. This hypothesis suggests that that after an initial dispersal from Africa to Eurasia at ~17Ma and subsequent radiation from Spain to China, fossil apes disperse back to Africa at least once and found the African ape and human lineage in the late Miocene. The purpose of this study is to test the Eurasian origin hypothesis through the analysis of spatial and temporal patterns of distribution, in situ evolution, interprovincial and intercontinental dispersals of Eurasian terrestrial mammals in response to environmental factors. Using the NOW and Paleobiology databases, together with data collected through survey and excavation of middle and late Miocene vertebrate localities in Hungary and Romania, taphonomic bias and sampling completeness of Eurasian faunas are assessed. -

(Suidae, Artiodactyla) from the Upper Miocene of Hayranlı-Haliminhanı, Turkey
Turkish Journal of Zoology Turk J Zool (2013) 37: 106-122 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-1202-4 Microstonyx (Suidae, Artiodactyla) from the Upper Miocene of Hayranlı-Haliminhanı, Turkey 1 2 3, Jan VAN DER MADE , Erksin GÜLEÇ , Ahmet Cem ERKMAN * 1 Spanish National Research Council, National Museum of Natural Sciences, Madrid, Spain 2 Department of Anthropology, Faculty of Languages, History, and Geography, Ankara University, Ankara, Turkey 3 Department of Anthropology, Faculty of Science and Literature, Ahi Evran University, Kırşehir, Turkey Received: 05.02.2012 Accepted: 11.07.2012 Published Online: 24.12.2012 Printed: 21.01.2013 Abstract: The suid remains from localities 58-HAY-2 and 58-HAY-19 in the Late Miocene Derindere Member of the İncesu Formation in the Hayranlı-Haliminhanı area (Sivas, Turkey) are described and referred to as Microstonyx major (Gervais, 1848–1852). Microstonyx shows changes in incisor morphology, which are interpreted as a further adaptation to rooting. This occurred probably in a short period between 8.7 and 8.121 Ma ago and possibly is a reaction to environmental change. The incisor morphology in locality 58-HAY-2 suggests that it is temporally close to this change, which would imply that this locality and the lithostratigraphically lower 58-HAY-19 belong to the lower part of MN11 and not to MN12. The findings are discussed in the regional context and contribute to the knowledge of the Anatolian fossil mammals. Key words: Suidae, Microstonyx, rooting, ecology, Late Miocene 1. Introduction 1.1. Location and stratigraphy The first of the Hayranlı-Haliminhanı fossil localities was Anatolia lies at the intersection of Asia, Europe, and Afro- discovered in 1993 by members of the Vertebrate Fossils Arabia, and its geology has been subject to the plate tectonic Research Project, a collaborative survey effort. -

CONGENITAL MALFORMATIONS of the INNER EAR Malformaciones Congénitas Del Oído Interno
topic review CONGENITAL MALFORMATIONS OF THE INNER EAR Malformaciones congénitas del oído interno. Revisión de tema Laura Vanessa Ramírez Pedroza1 Hernán Darío Cano Riaño2 Federico Guillermo Lubinus Badillo2 Summary Key words (MeSH) There are a great variety of congenital malformations that can affect the inner ear, Ear with a diversity of physiopathologies, involved altered structures and age of symptom Ear, inner onset. Therefore, it is important to know and identify these alterations opportunely Hearing loss Vestibule, labyrinth to lower the risks of all the complications, being of great importance, among others, Cochlea the alterations in language development and social interactions. Magnetic resonance imaging Resumen Existe una gran variedad de malformaciones congénitas que pueden afectar al Palabras clave (DeCS) oído interno, con distintas fisiopatologías, diferentes estructuras alteradas y edad Oído de aparición de los síntomas. Por lo anterior, es necesario conocer e identificar Oído interno dichas alteraciones, con el fin de actuar oportunamente y reducir el riesgo de las Pérdida auditiva Vestíbulo del laberinto complicaciones, entre otras —de gran importancia— las alteraciones en el área del Cóclea lenguaje y en el ámbito social. Imagen por resonancia magnética 1. Epidemiology • Hyperbilirubinemia Ear malformations occur in 1 in 10,000 or 20,000 • Respiratory distress from meconium aspiration cases (1). One in every 1,000 children has some degree • Craniofacial alterations (3) of sensorineural hearing impairment, with an average • Mechanical ventilation for more than five days age at diagnosis of 4.9 years. The prevalence of hearing • TORCH Syndrome (4) impairment in newborns with risk factors has been determined to be 9.52% (2). -

Geodiversitas 2020 42 8
geodiversitas 2020 42 8 né – Car ig ni e vo P r e e s n a o f h t p h é e t S C l e a n i r o o z o m i e c – M DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEUR EN CHEF / EDITOR-IN-CHIEF : Didier Merle ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Emmanuel Côtez ([email protected]) MISE EN PAGE / PAGE LAYOUT : Emmanuel Côtez COMITÉ SCIENTIFIQUE / SCIENTIFIC BOARD : Christine Argot (Muséum national d’Histoire naturelle, Paris) Beatrix Azanza (Museo Nacional de Ciencias Naturales, Madrid) Raymond L. Bernor (Howard University, Washington DC) Alain Blieck (chercheur CNRS retraité, Haubourdin) Henning Blom (Uppsala University) Jean Broutin (Sorbonne Université, Paris) Gaël Clément (Muséum national d’Histoire naturelle, Paris) Ted Daeschler (Academy of Natural Sciences, Philadelphie) Bruno David (Muséum national d’Histoire naturelle, Paris) Gregory D. Edgecombe (The Natural History Museum, Londres) Ursula Göhlich (Natural History Museum Vienna) Jin Meng (American Museum of Natural History, New York) Brigitte Meyer-Berthaud (CIRAD, Montpellier) Zhu Min (Chinese Academy of Sciences, Pékin) Isabelle Rouget (Muséum national d’Histoire naturelle, Paris) Sevket Sen (Muséum national d’Histoire naturelle, Paris) Stanislav Štamberg (Museum of Eastern Bohemia, Hradec Králové) Paul Taylor (The Natural History Museum, Londres) COUVERTURE / COVER : Made from the Figures of the article. Geodiversitas est indexé dans / Geodiversitas is indexed in: – Science Citation Index Expanded (SciSearch®) – ISI Alerting -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

A New Species of Chleuastochoerus (Artiodactyla: Suidae) from the Linxia Basin, Gansu Province, China
Zootaxa 3872 (5): 401–439 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3872.5.1 http://zoobank.org/urn:lsid:zoobank.org:pub:5DBE5727-CD34-4599-810B-C08DB71C8C7B A new species of Chleuastochoerus (Artiodactyla: Suidae) from the Linxia Basin, Gansu Province, China SUKUAN HOU1, 2, 3 & TAO DENG1 1Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences Beijing 100044, China 2State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sci- ences Nanjing 210008, China 3Corresponding author. E-mail: [email protected] Abstract The Linxia Basin, Gansu Province, China, is known for its abundant and well-preserved fossils. Here a new species, Chleuastochoerus linxiaensis sp. nov., is described based on specimens collected from the upper Miocene deposits of the Linxia Basin, distinguishable from C. stehlini by the relatively long facial region, more anteromedial-posterolaterally compressed upper canine and more complicated cheek teeth. A cladistics analysis placed Chleuastochoerus in the sub- family Hyotheriinae, being one of the basal taxa of this subfamily. Chleuastochoerus linxiaensis and C. stehlini are con- sidered to have diverged before MN 10. C. tuvensis from Russia represents a separate lineage of Chleuastochoerus, which may have a closer relationship to C. stehlini but bears more progressive P4/p4 and M3. Key words: Linxia Basin, upper Miocene Liushu Formation, Hyotheriinae, Chleuastochoerus, phylogeny Introduction Chleuastochoerus Pearson 1928 is a small late Miocene-early Pliocene fossil pig (Suidae). -

Abstract Volume 9Th Swiss Geoscience Meeting Zurich, 11Th – 13Th November 2011
Abstract Volume 9th Swiss Geoscience Meeting Zurich, 11th – 13th November 2011 14. Traces of life on planet Earth: A tribute to the late Professor Lukas Hottinger 306 14. Traces of life on planet Earth: A tribute to the late Professor Lukas Hottinger Lionel Cavin, Michael Hautmann “Schweizerische Paläontologische Gesellschaft” (SPG/SPS) “Kommission der Schweizerischen Paläontologischen Abhandlungen” (KSPA) TALKS: Symposium 14: Traces of life on planet Earth Symposium 14: Traces 14.1 Geiger, M., Wilson, L. A. B., Costeur, L., Scheyer, T. M., Aguilera, O. A., Sánchez-Villagra, M. R.: Giant rodents from the northern Neotropics - taxonomic, phylogenetic and developmental aspects of their evolution within the caviomorph radiation 14.2 Hiard F., Mennecart B., Berger J.-P.: Palaeoenvironmental reconstruction of the Swiss Molasse Basin (Oligocene and Early Miocene) on the basis of postcranial remains of ruminants (Artiodactyla, Mammalia) 14.3 Hofmann R., Hautmann M., Bucher H.: Ecological structure and taxa distribution in near shore habitats of the Virgin Formation (south-western Utah): Implications for the Early Triassic recovery 14.4 Kolb, C.: Growth patterns deduced from bone histology of the dwarfed island deer Candiacervus from the Late Pleistocene of Crete 14.5 Mary Y., Knappertsbusch M.: Biogeographic morphological investigation of menardiform globorotalids in a time slice at 3.2 My (Mid-Pliocene) 14.6 Meister P.H.: Was the Triassic a plumeworld? 14.7 Mennecart B.: Was Europe an evolutionary DEAD END? Case of the Oligocene-Early Miocene -

The Temporal Bone
Anatomic Moment The Temporal Bone Joel D. Swartz, David L. Daniels, H. Ric Harnsberger, Katherine A. Shaffer, and Leighton Mark Despite the advent of thin-section high-reso- the inner ear structures and the external lution computed tomography and, more re- environment. cently, unique magnetic resonance imaging se- Virtually all textbooks define the inner ear as quences with thin sections, T2 weighting, and a membranous labyrinth housed within an os- maximum-intensity projection techniques, seous labyrinth. The membranous labyrinth three-dimensional neuroanatomy of the tempo- contains endolymph, a fluid rich in potassium ral bone and related structures remains some- and low in sodium, similar to intracellular fluid. what of an enigma to many medical specialists, Interposed between the membranous labyrinth neuroradiologists included. Therefore, we are and the osseous labyrinth resides a supportive undertaking a series of anatomic moments in perilymphatic labyrinth. Perilymph is similar to the hope of solidifying the most important ana- cerebrospinal fluid and other extracellular tissue tomic concepts as they relate to this region. Our fluid. approach will be organized so as to consider the The osseous labyrinth consists of the bony temporal bone to represent the complex inter- edifice for the vestibule, semicircular canals, relationship of three systems: hearing, balance, cochlea, and vestibular aqueduct. The mem- and related neuroanatomic and neurovascular branous labyrinth consists of the utricle and structures. saccule (located within the vestibule), the semi- The ear originated in fish as a water motion circular ducts, the cochlear duct, and the en- detection system (1). The vestibular (balance) dolymphatic duct. The latter is a channel within mechanism becomes more complex as we the vestibular aqueduct with communications to scale the embryologic ladder and endolymph the utricle and saccule. -
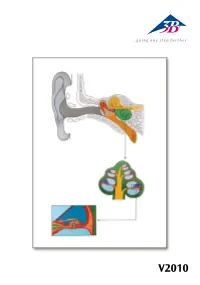
B005DTJC8Y.Usermanual.Pdf
V2010 Auris Latin Auris externa 1 Auricula 2 Meatus acusticus externus 3 Membrana tympanica Auris media 4 Ossicula auditus: 4 a Malleus 4 b Incus 4 c Stapes 5 Cavitas tympani 6 Tuba auditiva 7 Fenestra vestibuli 8 Fenestra cochleae Auris interna 9 Labyrinthus osseus: 9 a Canalis semicircularis posterior 9 b Canalis semicircularis lateralis 9 c Canalis semicircularis anterior 9 d Vestibulum 9 e Cochlea 10 N. vestibularis 11 N. cochlearis ® 12 N. vestibulocochlearis [VIII] 13 Lig. spirale 14 Scala vestibuli 15 Ductus cochlearis 16 Scala tympani 17 N. cochlearis 18 Ganglion cochleare 19 Organum spirale 20 Modiolus cochleae 21 Paries vestibularis 22 Sulcus spiralis internus 23 Membrana tectoria 24 Cuniculus medius 25 Cuniculus externus 26 Cellulae terminales externae 27 Cellulae sustentaculares externae 28 Sulcus spiralis externus 29 Lamina basilaris 30 Cellulae phalangea externae 31 Cellulae capillares externae 32 Cellula stela externa 33 Cuniculus internus 34 Cellula capillaris interna 35 Cellula stela interna 36 Lamina spiralis ossea 37 Limbus spiralis English The Ear A Sectional view of the human ear B Sectional view of cochlea C Sectional view of cochlear duct with spiral organ External ear 1 Auricle 2 External acoustic meatus 3 Tympanic membrane Middle ear 4 Auditory ossicles: 4 a Malleus 4 b Incus 4 c Stapes 5 Tympanic cavity 6 Pharyngotympanice tube 7 Oval window 8 Round window Internal ear 9 Bony labyrinth 9 a Posterior semicircular canal 9 b Lateral semicircular canal 9 c Anterior semicircular canal ® 9 d Vestibule 9 e Cochlea 10