Immunologic Aspects of Parasitic Infections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Case 16-2019: a 53-Year-Old Man with Cough and Eosinophilia
The new england journal of medicine Case Records of the Massachusetts General Hospital Founded by Richard C. Cabot Eric S. Rosenberg, M.D., Editor Virginia M. Pierce, M.D., David M. Dudzinski, M.D., Meridale V. Baggett, M.D., Dennis C. Sgroi, M.D., Jo-Anne O. Shepard, M.D., Associate Editors Alyssa Y. Castillo, M.D., Case Records Editorial Fellow Emily K. McDonald, Sally H. Ebeling, Production Editors Case 16-2019: A 53-Year-Old Man with Cough and Eosinophilia Rachel P. Simmons, M.D., David M. Dudzinski, M.D., Jo-Anne O. Shepard, M.D., Rocio M. Hurtado, M.D., and K.C. Coffey, M.D. Presentation of Case From the Department of Medicine, Bos- Dr. David M. Dudzinski: A 53-year-old man was evaluated in an urgent care clinic of ton Medical Center (R.P.S.), the Depart- this hospital for 3 months of cough. ment of Medicine, Boston University School of Medicine (R.P.S.), the Depart- Five years before the current evaluation, the patient began to have exertional ments of Medicine (D.M.D., R.M.H.), dyspnea and received a diagnosis of hypertrophic obstructive cardiomyopathy, with Radiology (J.-A.O.S.), and Pathology a resting left ventricular outflow gradient of 110 mm Hg on echocardiography. (K.C.C.), Massachusetts General Hos- pital, and the Departments of Medicine Although he received medical therapy, symptoms persisted, and percutaneous (D.M.D., R.M.H.), Radiology (J.-A.O.S.), alcohol septal ablation was performed 1 year before the current evaluation, with and Pathology (K.C.C.), Harvard Medical resolution of the exertional dyspnea. -
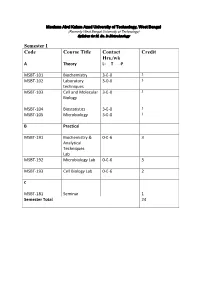
Syllabus for M
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -

~.. R---'------ : KASMERA: Vol
~.. r---'-------------- : KASMERA: Vol.. 9, No. 1 4,1981 Zulla. Maracaibo. Venezuela. PROTOZOOS DE VENEZUELA Carlos Diaz Ungrla· Tratamos con este trabajo de ofrecer una puesta al día de los protozoos estudiados en nuestro país. Con ello damos un anticipo de lo que será nuestra próxima obra, en la cual, además de actualizar los problemas taxonómicos, pensamos hacer énfasis en la ultraestructura, cuyo cono cimiento es básico hoy día para manejar los protozoos, comQ animales unicelulares que son. Igualmente tratamos de difundir en nuestro medio la clasificación ac tual, que difiere tanto de la que se sigue estudiando. y por último, tratamos de reunir en un solo trabajo toda la infor mación bibliográfica venezolana, ya que es sabido que nuestros autores se ven precisados a publicar en revistas foráneas, y esto se ha acentuado en los últimos diez (10) años. En nuestro trabajo presentaremos primero la lista alfabética de los protozoos venezolanos, después ofreceremos su clasificación, para terminar por distribuirlos de acuerdo a sus hospedadores . • Profesor de la Facultad de Ciencias Veterinarias de la Universidad del Zulia. Maracaibo-Venezuela. -147 Con la esperanza de que nuestro trabajo sea útil anuestros colegas. En Maracaibo, abril de mil novecientos ochenta. 1 LISTA ALF ABETICA DE LOS PROTOZOOS DE VENEZUELA Babesia (Babesia) bigemina, Smith y Kilbome, 1893. Seflalada en Bos taurus por Zieman (1902). Deutsch. Med. Wochens., 20 y 21. Babesia (Babesia) caballi Nuttall y Stricldand. 1910. En Equus cabal/uso Gallo y Vogelsang (1051). Rev. Med.Vet. y Par~. 10 (1-4); 3. Babesia (Babesia) canis. Piana y Galli Valerio, 1895. En Canis ¡ami/iaris. -

Plasmodium Scientific Classification
Plasmodium - Wikipedia https://en.wikipedia.org/wiki/Plasmodium From Wikipedia, the free encyclopedia Plasmodium is a genus of parasitic alveolates, many of which cause malaria in their hosts.[1] The parasite always has two hosts in its life Plasmodium cycle: a Dipteran insect host and a vertebrate host. Sexual reproduction always occurs in the insect, making it the definitive host.[2] The life-cycles of Plasmodium species involve several different stages both in the insect and the vertebrate host. These stages include sporozoites, which are injected by the insect vector into the vertebrate host's blood. Sporozoites infect the host liver, giving rise to merozoites and (in some species) hypnozoites. These move into the blood where they infect red blood cells. In the red blood cells, the parasites can either form more merozoites to infect more red blood cells, or produce gametocytes which are taken up by insects which feed on the vertebrate host. In the insect host, gametocytes merge to sexually reproduce. After sexual reproduction, parasites grow into new sporozoites, which move to the insect's salivary glands, from which they can infect a vertebrate False-colored electron micrograph of a [1] host bitten by the insect. Plasmodium sp. sporozoite. The genus Plasmodium was first described in 1885. It now contains Scientific classification about 200 species, which are spread across the world where both the (unranked): SAR insect and vertebrate hosts are present. Five species regularly infect humans, while many others infect birds, reptiles, -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

Disseminated Peritoneal Schistosoma Japonicum: a Case Report And
[Downloaded free from http://www.saudiannals.net on Monday, May 10, 2010] case report Disseminated peritoneal Schistosoma japonicum: a case report and review of the pathological manifestations of the helminth Salah Al-Waheeb,a Maryam Al-Murshed,a Fareeda Dashti,b Parsotam R. Hira,c Lamia Al-Sarrafd From the aDepartments of Histopathology, and bSurgery, Mubarak Al-Kabeer Hospital, cDepartment of Microbiology, Kuwait University, dDepart- ment of Radiology, Mubarak Al-Kabeer Hospital, Jabriyah, Kuwait Correspondence: Salah Al-Waheeb, MD · Mubarak Al-Kabeer Hospital, PO Box 72, Code 71661, Jabriyah, Shamiyah City, Kuwait · T: +975-531- 2700 ext. 2188 · [email protected] · Approved for publication August 2008 Ann Saudi Med 2009; 29(2): 149-152 Schistosomiasis (also known as bilharzia, bilharziasis, bilharziosis or snail fever) is a human disease syn- drome caused by infection from one of several species of parasitic trematodes of the genus Schistosoma. The three main species infecting humans are S haematobium, S japonicum, and S mansoni. S japonicum is most common in the far east, mostly in China and the Philippines. We present an unusual case of S japonicum in a 32-year-old Filipino woman who had schistosomal ova studding the peritoneal cavity and forming a mass in the right iliac fossa. chistosomiasis (also known as bilharzia, bilharziaa liver (Figure 1). CT examination showed multiple cala asis, bilharziosis or snail fever) is a human disease cific foci throughout the abdomen, particularly in the Ssyndrome caused by infection from one of several RIF. Prominent small bowel dilatation and fluid colleca species of parasitic trematodes of the genus Schistosoma. -

Ultrasound of Tropical Medicine Parasitic Diseases of the Liver
Ultrasound of the liver …. 20.11.2012 11:05 1 EFSUMB – European Course Book Editor: Christoph F. Dietrich Ultrasound of Tropical Medicine Parasitic diseases of the liver Enrico Brunetti1, Tom Heller2, Francesca Tamarozzi3, Adnan Kabaalioglu4, Maria Teresa Giordani5, Joachim Richter6, Roberto Chiavaroli7, Sam Goblirsch8, Carmen Cretu9, Christoph F Dietrich10 1 Department of Infectious Diseases, San Matteo Hospital Foundation- University of Pavia, Pavia, Italy 2 Department of Internal Medicine, Klinikum Muenchen Perlach, Munich, Germany 3 Department of Infectious Diseases, San Matteo Hospital Foundation- University of Pavia, Pavia, Italy 4 Department of Radiology, Akdeniz University, Antalya, Turkey 5 Infectious and Tropical Diseases Unit, San Bortolo Hospital, Vicenza, Italy 6 Tropenmedizinische Ambulanz, Klinik für Gastroenterologie, Hepatologie und Infektiologie, Heinrich-Heine-Universität, Düsseldorf, Germany 7 Infectious Diseases Unit, Santa Caterina Novella Hospital, Galatina, Italy 8 Department of Medicine and Pediatrics, University of Minnesota, Minneapolis, MN, USA 9 University of Medicine and Pharmacy "Carol Davila" Parasitology Department Colentina Teaching Hospital, Bucharest, Romania 10 Caritas-Krankenhaus Bad Mergentheim, Germany Ultrasound of parasitic disease …. 20.11.2012 11:05 2 Content Content ....................................................................................................................................... 2 Amoebiasis ................................................................................................................................ -

New Aspects of Human Trichinellosis: the Impact of New Trichinella Species F Bruschi, K D Murrell
15 REVIEW Postgrad Med J: first published as 10.1136/pmj.78.915.15 on 1 January 2002. Downloaded from New aspects of human trichinellosis: the impact of new Trichinella species F Bruschi, K D Murrell ............................................................................................................................. Postgrad Med J 2002;78:15–22 Trichinellosis is a re-emerging zoonosis and more on anti-inflammatory drugs and antihelminthics clinical awareness is needed. In particular, the such as mebendazole and albendazole; the use of these drugs is now aided by greater clinical description of new Trichinella species such as T papuae experience with trichinellosis associated with the and T murrelli and the occurrence of human cases increased number of outbreaks. caused by T pseudospiralis, until very recently thought to The description of new Trichinella species, such as T murrelli and T papuae, as well as the occur only in animals, requires changes in our handling occurrence of outbreaks caused by species not of clinical trichinellosis, because existing knowledge is previously recognised as infective for humans, based mostly on cases due to classical T spiralis such as T pseudospiralis, now render the clinical picture of trichinellosis potentially more compli- infection. The aim of the present review is to integrate cated. Clinicians and particularly infectious dis- the experiences derived from different outbreaks around ease specialists should consider the issues dis- the world, caused by different Trichinella species, in cussed in this review when making a diagnosis and choosing treatment. order to provide a more comprehensive approach to diagnosis and treatment. SYSTEMATICS .......................................................................... Trichinellosis results from infection by a parasitic nematode belonging to the genus trichinella. -

Biliary Obstruction Caused by the Liver Fluke, Fasciola Hepatica
CME Practice CMAJ Cases Biliary obstruction caused by the liver fluke, Fasciola hepatica Takuya Ishikawa MD PhD, Vanessa Meier-Stephenson MD PhD, Steven J. Heitman MD MSc Competing interests: None 20-year-old previously healthy man declared. presented to hospital with a two-day This article has been peer A history of right upper quadrant pain reviewed. and vomiting. Nine months earlier, he had The authors have obtained immigrated to Canada from Sudan, but he had patient consent. also lived in Djibouti and Ethiopia. Four Correspondence to: months before he presented to hospital, he Steven Heitman, received a diagnosis of tuberculous lymphade- [email protected] nitis and a four-drug course of tuberculosis CMAJ 2016. DOI:10.1503 treatment was started. However, he was non- /cmaj.150696 adherent after only two months of treatment. In addition, results from screening tests at that time showed evidence of schistosomiasis for Figure 1: A flat, leaf-shaped, brown worm emerg- which he was prescribed praziquantel. ing from the common bile duct of a 20-year-old On examination, he was alert and without man with abdominal pain. jaundice or scleral icterus. He had right upper quadrant tenderness on abdominal examination, ter of 1.1 cm. A computed tomography scan of but there were no palpable masses. The remain- the abdomen also showed prominence of the der of his examination was unremarkable. Labo- common bile duct, but no calcified stone was ratory test results showed elevated liver enzymes identified (Appendix 1). A hepatobiliary imino- (aspartate transaminase 133 [normal < 40] U/L, diacetic acid scan suggested distal obstruction in alanine transaminase 217 [normal < 41] U/L, the common bile duct. -

Imaging Parasitic Diseases
Insights Imaging (2017) 8:101–125 DOI 10.1007/s13244-016-0525-2 REVIEW Unexpected hosts: imaging parasitic diseases Pablo Rodríguez Carnero1 & Paula Hernández Mateo2 & Susana Martín-Garre2 & Ángela García Pérez3 & Lourdes del Campo1 Received: 8 June 2016 /Revised: 8 September 2016 /Accepted: 28 September 2016 /Published online: 23 November 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Radiologists seldom encounter parasitic dis- • Some parasitic diseases are still endemic in certain regions eases in their daily practice in most of Europe, although in Europe. the incidence of these diseases is increasing due to mi- • Parasitic diseases can have complex life cycles often involv- gration and tourism from/to endemic areas. Moreover, ing different hosts. some parasitic diseases are still endemic in certain • Prompt diagnosis and treatment is essential for patient man- European regions, and immunocompromised individuals agement in parasitic diseases. also pose a higher risk of developing these conditions. • Radiologists should be able to recognise and suspect the This article reviews and summarises the imaging find- most relevant parasitic diseases. ings of some of the most important and frequent human parasitic diseases, including information about the para- Keywords Parasitic diseases . Radiology . Ultrasound . site’s life cycle, pathophysiology, clinical findings, diag- Multidetector computed tomography . Magnetic resonance nosis, and treatment. We include malaria, amoebiasis, imaging toxoplasmosis, trypanosomiasis, leishmaniasis, echino- coccosis, cysticercosis, clonorchiasis, schistosomiasis, fascioliasis, ascariasis, anisakiasis, dracunculiasis, and Introduction strongyloidiasis. The aim of this review is to help radi- ologists when dealing with these diseases or in cases Parasites are organisms that live in another organism at the where they are suspected. -

Learning Objectives
Restrictive Lung Diseases & Pulmonary Vascular Disease Pulmonary 2018 RESTRICTIVE LUNG DISEASES AND PULMONARY VASCULAR DISEASE Joel Thibodeaux, MD, Phone: 469-419-4535 Email: [email protected] INTRODUCTION This lecture will discuss basic concepts, etiologic factors, pathologic features, pathogenesis, and clinicopathologic findings in the different types of interstitial lung diseases. It also will touch briefly on pulmonary vascular diseases. LEARNING OBJECTIVES: • Acute respiratory distress syndrome (ARDS) (BP, pp. 460-461) o Define ARDS and list common causes o Illustrate the mechanism of lung injury in ARDS and the role of proinflammatory and anti-inflammatory mediators o Describe the morphologic features of ARDS. Understand how they evolve, and know what happens if the patient survives. • Diffuse interstitial lung disease (BP, pp. 472-474, 480-482) o List the major forms of diffuse interstitial lung diseases discussed o Be able to identify classic morphologic features of common interstitial lung diseases. o Recognize the major causes of pneumoconioses (BP, pp. 474-478) o Delineate the gross and microscopic features resulting from exposure to coal dust, silica, organic/animal dust, and asbestos. o Sarcoidosis (BP, pp. 478-480) . Define sarcoidosis . List the most common organs involved, and describe the characteristic histologic lesion . Describe the radiographic, gross, and histologic appearance of lesions in the hilar lymph nodes and lungs • Define primary pulmonary hypertension. Recognize the characteristic histologic -

A Meta-Analysis of the Genus Alouatta
Chapter 17 Ecological and Anthropogenic Influences on Patterns of Parasitism in Free-Ranging Primates: A Meta-analysis of the Genus Alouatta Martin M. Kowalewski and Thomas R. Gillespie 17.1 Introduction Parasites play a central role in tropical ecosystems, affecting the ecology and evolution of species interactions, host population growth and regulation, and com- munity biodiversity (Esch and Fernandez 1993; Hudson, Dobson and Newborn 1998; Hochachka and Dhondt 2000; Hudson et al. 2002). Our understanding of how nat- ural and anthropogenic factors affect host-parasite dynamics in free-ranging pri- mate populations (Gillespie, Chapman and Greiner 2005a; Gillespie, Greiner and Chapman 2005b; Gillespie and Chapman 2006) and the relationship between wild primates and human health in rural or remote areas (McGrew et al. 1989; Stuart et al. 1990; Muller-Graf, Collins and Woolhouse 1997; Gillespie et al. 2005b; Pedersen et al. 2005) remain largely unexplored. The majority of emerging infec- tious diseases are zoonotic – easily transferred among humans, wildlife, and domes- ticated animals – (Nunn and Altizer 2006). For example, Taylor, Latham and Woolhouse (2001) found that 61% of human pathogens are shared with animal hosts. Identifying general principles governing parasite occurrence and prevalence is critical for planning animal conservation and protecting human health (Nunn et al. 2003). In this review, we examine how various ecological and anthropogenic factors affect patterns of parasitism in free-ranging howler monkeys (Genus Alouatta). 17.1.1 Evidence of the Relationships Between Howlers and Parasitic Diseases in South America The genus Alouatta is the most geographically widespread non-human primate in South America, with 8 of 10 Alouatta species ranging from Northern Colombia M.M.