Ips International Guidelines for the Acquisition, Care and Breeding of Nonhuman Primates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Non-Human Primates in Research in Primates Non-Human of Use The
The use of non-human primates in research The use of non-human primates in research A working group report chaired by Sir David Weatherall FRS FMedSci Report sponsored by: Academy of Medical Sciences Medical Research Council The Royal Society Wellcome Trust 10 Carlton House Terrace 20 Park Crescent 6-9 Carlton House Terrace 215 Euston Road London, SW1Y 5AH London, W1B 1AL London, SW1Y 5AG London, NW1 2BE December 2006 December Tel: +44(0)20 7969 5288 Tel: +44(0)20 7636 5422 Tel: +44(0)20 7451 2590 Tel: +44(0)20 7611 8888 Fax: +44(0)20 7969 5298 Fax: +44(0)20 7436 6179 Fax: +44(0)20 7451 2692 Fax: +44(0)20 7611 8545 Email: E-mail: E-mail: E-mail: [email protected] [email protected] [email protected] [email protected] Web: www.acmedsci.ac.uk Web: www.mrc.ac.uk Web: www.royalsoc.ac.uk Web: www.wellcome.ac.uk December 2006 The use of non-human primates in research A working group report chaired by Sir David Weatheall FRS FMedSci December 2006 Sponsors’ statement The use of non-human primates continues to be one the most contentious areas of biological and medical research. The publication of this independent report into the scientific basis for the past, current and future role of non-human primates in research is both a necessary and timely contribution to the debate. We emphasise that members of the working group have worked independently of the four sponsoring organisations. Our organisations did not provide input into the report’s content, conclusions or recommendations. -

Non-Human Primates in Biomedical Research
Science Shop for Biology Non-human primates in biomedical research Frouke Pieters P-UB-2006-06 Non-human primates in biomedical research Reasons and alternatives for their use Frouke Pieters Science shop for Biology Netherlands Centre Alternatives to Animal Use, Utrecht University, The Netherlands April 2007 P-UB-2006-06 Colofon Report number P-UB-2006-06 ISBN 978-90-5209-158-7 Price € 5,- Publication date April 2007 Edition First Title Non-human primates in biomedical research Reasons and alternatives for their use Author Frouke Pieters Supervisor Prof. Dr. C. Hendriksen, Netherlands Centre Alternatives to animal use, Utrecht University, The Netherlands Project coordinator Ir. M. A. Vaal, Science Shop for Biology, Utrecht University Commissioned by Working party 'Primate Research in the Netherlands' under the auspices of the Dutch Association for Laboratory Animal Science, Amsterdam Reproduction Document Diensten Centrum Uithof Publisher Science shop for Biology, Utrecht University Padualaan 8, 3584 CH Utrecht, The Netherlands. .. 31 30 253 7363 www.bio.uu.nl/scienceshop Copyright This document (or parts thereof) may not be multiplied in any form. Parts of the document may be used for other publications, if a reference is included. Contents Preface 5 Summary 7 Samenvatting 8 1 Introduction 9 1.1 Motivation 9 1.2 Definition of the problem 10 1.3 Scope and definitions 10 1.4 Approach 11 1.5 Structure 12 2 Use of primates 13 2.1 Figures 13 2.2 Goals of primate studies 18 2.3 Regulations on animal experimentation 21 2.4 Ethics 22 2.5 Problems -
![Environmental Enrichment for Nonhuman Primates Resource Guide [Electronic Resource] AWIC Resource Series No](https://docslib.b-cdn.net/cover/5536/environmental-enrichment-for-nonhuman-primates-resource-guide-electronic-resource-awic-resource-series-no-915536.webp)
Environmental Enrichment for Nonhuman Primates Resource Guide [Electronic Resource] AWIC Resource Series No
United States Department of Agriculture Environmental Enrichment Agricultural Research Service for Nonhuman Primates National Agricultural Library Resource Guide Animal Welfare Information Center 2006 (Updated October 2009) Photo courtesy Photos8.com AWIC Resource Series No. 32 United States Department of Environmental Agriculture Enrichment for Agricultural Research Service Nonhuman Primates National Agricultural Resource Guide Library AWIC Resource Series No. 32 Animal Welfare Information Center 2006 (Updated October 2009) Compiled by: Kristina M. Adams, M.S. Animal Welfare Information Center National Agricultural Library U.S. Department of Agriculture Beltsville, Maryland 20705 E-mail: [email protected] Web site: http://awic.nal.usda.gov Available online: http://www.nal.usda.gov/awic/pubs/Primates2009/primates.shtml National Agricultural Library Cataloging Record Adams, Kristina M. Environmental Enrichment for Nonhuman Primates Resource Guide [electronic resource] AWIC Resource Series No. 32, Updated 1. Environmental enrichment (Animal culture) -- Bibliography. 2. Primates -- Environmental Enrichment -- Bibliography. I. Animal Welfare Information Center (U.S.) II. Title. aHV4701 .A94 no. 32, Updated Disclaimers The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or a part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). -

Accountability
ACCOUNTABILITY animal experiments & freedom of information The assessment of projects under the Animals (Scientific Procedures) Act 1986 The licensing process The Animal Procedures Committee The application of Nolan principles ACCOUNTABILITY animal experiments & freedom of information - a parliamentary briefing CONTENTS 1. Introduction 1 2. Background 2 3. Secrecy vs Transparency 5 4. Put it to the test 9 5. The Animal Procedures Committee 13 6. Reform of the APC 16 7. Local Ethics Committees 21 8. Conclusions 25 Appendix: Profile of current members of the APC 261 Goldhawk Road, London W12 9PE. Tel. 0181 846 9777 Fax. 0181 846 9712 e-mail: [email protected] Web: http://www.cygnet.co.uk/navs ©NAVS 1997 ACCOUNTABILITY 1. Introduction There is undoubtedly considerable public disquiet that cruel, unnecessary or repetitive research continues on animals in British laboratories. Bland government assurances that our legislation is the ‘best in the world’ do not convince a public now familiar with video and photographic evidence of the reality of animal experimentation. The secrecy with which the law is administered only hardens the conviction that there is something to hide. Well documented evidence from the NAVS and others has shown that government guidelines and the ‘Code of Practice for the Housing and Care of Laboratory Animals’ are not diligently enforced and that the Home Office leans towards protection of vivisection industry interests rather than towards serving the public will. It has taken undercover investigations to expose serious abuses within the system. In March 1997 a Channel 4 investigation led to the threat of the revocation of the Certificate of Designation for Huntingdon Life Sciences and the prosecution of former staff members. -

Platforms and Funds for Alternatives to Animal Experimentation
Platforms and Funds for Alternatives to Animal Experimentation Live Kleveland A report from The Norwegian Reference Centre for Laboratory Animal Science & Alternatives, Norwegian School of Veterinary Science, Oslo, Norway 2005 ISBN 82-7725-120-3 This is a revised version of the report, after circulation to representatives of all ecopa platforms for approval. CONTENTS INTRODUCTION _____________________________________________________ 3 ACKNOWLEDGEMENTS ______________________________________________ 4 ECOPA AND EUROPEAN CONSENSUS-PLATFORMS FOR ALTERNATIVES TO ANIMAL EXPERIMENTATION _____________________________________ 5 Austria ______________________________________________________________ 5 Belgium _____________________________________________________________ 6 The Czech Republic ____________________________________________________ 6 Finland______________________________________________________________ 7 Germany_____________________________________________________________ 8 Italy_________________________________________________________________ 8 The Netherlands ______________________________________________________ 9 Spain_______________________________________________________________ 10 Sweden _____________________________________________________________ 11 Switzerland__________________________________________________________ 12 The UK _____________________________________________________________ 13 SUMMARY OF CONSENSUS-PLATFORMS FOR ALTERNATIVES TO ANIMAL EXPERIMENTATION ________________________________________________ 15 FUNDING OF -

The Ethics of Research Involving Animals Published by Nuffield Council on Bioethics 28 Bedford Square London WC1B 3JS
The ethics of research involving animals Published by Nuffield Council on Bioethics 28 Bedford Square London WC1B 3JS Telephone: +44 (0)20 7681 9619 Fax: +44 (0)20 7637 1712 Email: [email protected] Website: http://www.nuffieldbioethics.org ISBN 1 904384 10 2 May 2005 To order a printed copy please contact the Nuffield Council or visit the website. © Nuffield Council on Bioethics 2005 All rights reserved. Apart from fair dealing for the purpose of private study, research, criticism or review, no part of the publication may be produced, stored in a retrieval system or transmitted in any form, or by any means, without prior permission of the copyright owners. Designed by dsprint / redesign 7 Jute Lane Brimsdown Enfield EN3 7JL Printed by Latimer Trend & Company Ltd Estover Road Plymouth PL6 7PY The ethics of research involving animals Nuffield Council on Bioethics Professor Sir Bob Hepple QC, FBA (Chairman) Professor Catherine Peckham CBE (Deputy Chairman) Professor Tom Baldwin Professor Margot Brazier OBE* Professor Roger Brownsword Professor Sir Kenneth Calman KCB FRSE Professor Peter Harper The Rt Reverend Richard Harries DD FKC FRSL Professor Peter Lipton Baroness Perry of Southwark** (up to March 2005) Professor Lord Raymond Plant Professor Martin Raff FRS (up to March 2005) Mr Nick Ross (up to March 2005) Professor Herbert Sewell Professor Peter Smith CBE Professor Dame Marilyn Strathern FBA Dr Alan Williamson FRSE * (co-opted member of the Council for the period of chairing the Working Party on the ethics of prolonging -

Guide for the Care and Use of Laboratory Animals, 8Th Edition
GUIDE FOR THE CARE AND USE OF LABORATORY ANIMALS Eighth Edition Committee for the Update of the Guide for the Care and Use of Laboratory Animals Institute for Laboratory Animal Research Division on Earth and Life Studies THE NATIONAL ACADEMIES PRESS 500 Fifth Street, NW Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Govern- ing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineer- ing, and the Institute of Medicine. The members of the Committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This study was supported by the Office of Extramural Research, Office of the Direc- tor, National Institutes of Health/Department of Health and Human Services under Contract Number N01-OD-4-2139 Task Order #188; the Office of Research Integrity, Department of Health and Human Services; the Animal and Plant Health Inspection Service, U.S. Department of Agriculture; Association for Assessment and Accreditation of Laboratory Animal Care International; American Association for Laboratory Animal Science; Abbott Fund; Pfizer; American College of Laboratory Animal Medicine; Ameri- can Society of Laboratory Animal Practitioners; Association of Primate Veternarians. Any opinions, findings, conclusions, or recommendations expressed in this pub- lication are those of the authors and do not necessarily reflect the views of the organizations or agencies that provided support for the project. The content of this publication does not necessarily reflect the views or policies of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. -

Animal Experimentation Is Justified
Animal Experimentation Is Justified Animal Experimentation Is Justified The Rights of Animals, 2004 Listen Stuart Derbyshire is an assistant professor at the University of Pittsburgh Medical Center and a contributor to Animal Experimentation: Good or Bad? Animal research has played a major part in the development of medicine, and will continue to do so. Yet scientists are becoming increasingly apologetic about their work. Regulations brought in to protect animals' welfare are hindering vital research. There is no 'middle ground' between animal research and a broader concern with animal welfare. Scientists who research with animals have made a moral choice—to put human life first. They should mount a robust defence of their work. Animal research has been an integral part of the development of modern medicine, has saved an incalculable number of lives, and prevents tremendous human suffering. Yet it continues to be an issue of major political controversy.... But where are the scientists in this debate? A strong case for more animal research could easily be made. Yet scientists appear increasingly apologetic about their actions. I would argue that scientists have made a series of disastrous tactical errors in dealing with the animal rights movement, and they continue to do so. Most of the errors have to do with trying to accommodate to the animal rightsmovement, or to reason with it and make compromises. Scientists on the Defensive The most widespread accommodation is the adoption of 'the three Rs', first proposed in 1959 following a report for the Universities Federation for Animal Welfare (UFAW). The three Rs are 'refinement', 'reduction' and 'replacement'. -

THE BOYD GROUP and ANIMAL EXPERIMENTATION a Case Study of Deliberation
THE BOYD GROUP AND ANIMAL EXPERIMENTATION A Case Study of Deliberation by Robert Garner Abstract This article is an account of the work of the Boyd Group, an informal grouping of stakeholders on both sides of the debate about animal experimentation formed in Britain in the early 1990s. It is an explorative case study which aims to map the opinion-forming processes of the participants of the Boyd Group, many of whom were interviewed by the author, in light of deliberative theory and with the intention of generating suggestions for improved democratic practices in representative bodies split by seemingly intractable moral differences. Not only is animal experimentation a policy issue involving acute moral conflict, but the Boyd Group is also a body made up of partisans representing organisations on both sides of the debate. Not surprisingly, the transformation of views predicted by some deliberative theorists has not occurred. However, deliberation within the Boyd Group has had the effect of softening some of the views and attitudes of the participants, has facilitated some compromises and provides a useful guide to the methods available to those wishing to manage moral conflict. Professor of Political Theory at the School of History, Politics and International Relations, University of Leicester. E-mail: [email protected]. The author would like to acknowledge the financial support of the Centre for Animals and Social Justice, and the provision of a period of research leave by the University of Leicester. 79 Global Journal of Animal Law, Vol 5, No 1 (2017) 1. Introduction This article consists of a case study of the Boyd Group (hereinafter BG), an informal grouping of stakeholders on both sides of the debate about animal experimentation formed in Britain in the early 1990s. -

Genetic Modification of Animals: Scientific and Ethical Issues
WellBeing International WBI Studies Repository 4-30-2019 Genetic Modification of Animals: Scientific and Ethical Issues Jarrod Bailey Cruelty Free International, [email protected] Follow this and additional works at: https://www.wellbeingintlstudiesrepository.org/geneclo Part of the Animal Studies Commons, Bioethics and Medical Ethics Commons, and the Other Genetics and Genomics Commons Recommended Citation Bailey, J. (2019). Genetic modification of animals: Scientific and ethical issues. In Animal Experimentation: Working Towards a Paradigm Change (pp. 443-479). Brill. https://doi.org/10.1163/ 9789004391192_020 This material is brought to you for free and open access by WellBeing International. It has been accepted for inclusion by an authorized administrator of the WBI Studies Repository. For more information, please contact [email protected]. Chapter 19 Genetic Modification of Animals: Scientific and Ethical Issues Jarrod Bailey Senior Research Scientist, Cruelty Free International, United Kingdom [email protected] 1 Introduction The scientific method demands a willingness to correct and integrate previ- ous knowledge, based on observable, empirical, measurable evidence and subject to laws of reasoning; yet, it has scarcely been applied to non-human animal (hereinafter referred to as animal) research. Nevertheless, animal use in science started declining in the mid 1970s, at least in the United King- dom, resulting in a drop in the number of animals used approaching 50% be- tween the mid-1970s and mid 1980s (UK Home Office, 2016)—perhaps a tacit admission of problematic species differences that render animals poor models for humans. This trend was, however, reversed with the advent of genetically modified (GM) animals, animals whose genetic material has been deliberately altered in some way by insertion, deletion, or substitution of dna. -
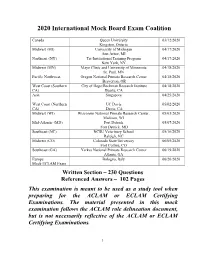
2020 International Mock Board Exam Coalition
2020 International Mock Board Exam Coalition Canada Queen University 03/12/2020 Kingston, Ontario Midwest (MI) University of Michigan 04/17/2020 Ann Arbor, MI Northeast (NY) Tri-Institutional Training Program 04/17/2020 New York, NY Midwest (MN) Mayo Clinic and University of Minnesota 04/18/2020 St. Paul, MN Pacific Northwest Oregon National Primate Research Center 04/18/2020 Beaverton, OR West Coast (Southern City of Hope/Beckman Research Institute 04/18/2020 CA) Duarte, CA Asia Singapore 04/25/2020 West Coast (Northern UC Davis 05/02/2020 CA) Davis, CA Midwest (WI) Wisconsin National Primate Research Center, 05/03/2020 Madison, WI Mid-Atlantic (MD) Fort Detrick 05/07/2020 Fort Detrick, MD Southeast (NC) NCSU Veterinary School 05/16/2020 Raleigh, NC Midwest (CO) Colorado State University 06/05/2020 Fort Collins, CO Southeast (GA) Yerkes National Primate Research Center 06/19/2020 Atlanta, GA Europe Bologna, Italy 06/26/2020 Mock ECLAM Exam Written Section – 230 Questions Referenced Answers – 102 Pages This examination is meant to be used as a study tool when preparing for the ACLAM or ECLAM Certifying Examinations. The material presented in this mock examination follows the ACLAM role delineation document, but is not necessarily reflective of the ACLAM or ECLAM Certifying Examinations. 1 2020 Exam Contributors Asia Bryan Emmett Ogden, DVM, DACLAM – Coordinator Mynn Michele Dy Varela, DVM Rex Malabanan Manguiat, DVM Jassia Pang, DVM, DACLAM Yasmina Arditi Paramastri, DVM, DACLAM Sharon Choy Heng Yee, BSc BVMS Canada Andrew Winterborn -

Report of the Animal Procedures Committee for 2004 HC
Report of the Animal Procedures Committee for 2004 Laid before Parliament by the Secretary of State for the Home Department pursuant to Section 20(5) of the Animals (Scientific Procedures) Act 1986, and on behalf of the Northern Ireland Minister of Health, Social Services and Public Safety pursuant to Section 20(5), as modified by Section 29, of the same Act. Ordered to be printed by the House of Commons 20 October 2005 LONDON: THE STATIONERY OFFICE HC 466 £7.75 Report of the Animal Procedures Committee for 2004 Laid before Parliament by the Secretary of State for the Home Department pursuant to Section 20(5) of the Animals (Scientific Procedures) Act 1986, and on behalf of the Northern Ireland Minister of Health, Social Services and Public Safety pursuant to Section 20(5), as modified by Section 29, of the same Act. Ordered to be printed by the House of Commons 20 October 2005 LONDON: THE STATIONERY OFFICE HC 466 £7.75 CONTENTS List of members at 31 December 2004 page iv Chairman’s Letter to the Rt Hon Charles Clarke MP, Secretary of State for the Home Department and to Shaun Woodward MP, the Northern Ireland Minister of Health, Social Services and Public Safety page v Introduction: page 1 The work of the Committee in 2004: Applications paragraph 1 Primates report and stakeholder forum paragraph 12 Other work of the Primates Sub-Committee paragraph 14 Research and Alternatives Sub-Committee and the establishment of NC3Rs paragraph 15 Education and Training Sub-Committee paragraph 30 Housing and Husbandry Sub-Committee paragraph 36 Review