Bombus Phylogeny
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Following the Cold
Systematic Entomology (2018), 43, 200–217 DOI: 10.1111/syen.12268 Following the cold: geographical differentiation between interglacial refugia and speciation in the arcto-alpine species complex Bombus monticola (Hymenoptera: Apidae) BAPTISTE MARTINET1 , THOMAS LECOCQ1,2, NICOLAS BRASERO1, PAOLO BIELLA3,4, KLÁRA URBANOVÁ5,6, IRENA VALTEROVÁ5, MAURIZIO CORNALBA7,JANOVE GJERSHAUG8, DENIS MICHEZ1 andPIERRE RASMONT1 1Laboratory of Zoology, Research Institute of Biosciences, University of Mons, Mons, Belgium, 2Research Unit Animal and Functionalities of Animal Products (URAFPA), University of Lorraine-INRA, Vandoeuvre-lès-Nancy, France, 3Faculty of Science, Department of Zoology, University of South Bohemia, Ceskéˇ Budejovice,ˇ Czech Republic, 4Biology Centre of the Academy of Sciences of the Czech Republic, v.v.i., Institute of Entomology, Ceskéˇ Budejovice,ˇ Czech Republic, 5Academy of Sciences of the Czech Republic, Institute of Organic Chemistry and Biochemistry, Prague, Czech Republic, 6Faculty of Tropical AgriSciences, Department of Sustainable Technologies, Czech University of Life Sciences, Prague, Czech Republic, 7Department of Mathematics, University of Pavia, Pavia, Italy and 8Norwegian Institute for Nature Research, Trondheim, Norway Abstract. Cold-adapted species are expected to have reached their largest distribution range during a part of the Ice Ages whereas postglacial warming has led to their range contracting toward high-latitude and high-altitude areas. This has resulted in an extant allopatric distribution of populations and possibly to trait differentiations (selected or not) or even speciation. Assessing inter-refugium differentiation or speciation remains challenging for such organisms because of sampling difficulties (several allopatric populations) and disagreements on species concept. In the present study, we assessed postglacial inter-refugia differentiation and potential speciation among populations of one of the most common arcto-alpine bumblebee species in European mountains, Bombus monticola Smith, 1849. -

Bumble Bees of the Western United States” by Jonathan Koch, James Strange, and Paul Williams (2012)
Adapted from the “Bumble Bees of the Western United States” by Jonathan Koch, James Strange, and Paul Williams (2012). Bumble bees are one of Wyoming’s most important and mild-mannered pollinators. There are more than 20 species in Wyoming, which you can often tell apart by the color patterns on their bodies. This guide shows color patterns for queen bees only, and some species can have multiple queen Bumble patterns! of Bees Areas in yellow indicate where each species Wyoming is found in Wyoming wyomingbiodiversity.org Black Tail Bumble Bee How can you tell Bombus melanopygus it's a bumble bee? Common Bumble bees are the largest bodied bees in Wyoming. Queens can be up to two inches long, but most queens and workers are somewhat smaller than that. They're very hairy all over their bodies, and carry pollen in “baskets” on their hind legs. Did you find a bumble bee? Submit your observation to the Xerces Society of Invertebrate Conservation's website: BumbleBeeWatch.org You can be a member of the citizen science community! Brown-Belted Bumble Bee California Bumble Bee Bombus griseocollis Bombus californicus Common Uncommon Central Bumble Bee Cuckoo Bumble Bee Bombus centralis Bombus insularis Common Common Fernald Cuckoo Bumble Bee Forest Bumble Bee Bombus fernaldae Bombus sylvicola Uncommon Uncommon Frigid Bumble Bee Fuzzy-Horned Bumble Bee Bombus frigidus Bombus mixtus Rare Common Half-Black Bumble Bee High Country Bumble Bee Bombus vagans Bombus balteatus Common Common Hunt’s Bumble Bee Morrison Bumble Bee Bombus huntii Bombus morrisoni Common Common Nevada Bumble Bee Red-Belted Bumble Bee Bombus nevadensis Bombus rufocinctus Common Common Suckley Cuckoo Bumble Bee Bombus suckleyi Red-Belted Bumble Bee, continued Uncommon Two-Form Bumble Bee Western Bumble Bee Bombus bifarius Bombus occidentalis Common Rare throughout much of its range, but common in Wyoming. -

Classification of the Apidae (Hymenoptera)
Utah State University DigitalCommons@USU Mi Bee Lab 9-21-1990 Classification of the Apidae (Hymenoptera) Charles D. Michener University of Kansas Follow this and additional works at: https://digitalcommons.usu.edu/bee_lab_mi Part of the Entomology Commons Recommended Citation Michener, Charles D., "Classification of the Apidae (Hymenoptera)" (1990). Mi. Paper 153. https://digitalcommons.usu.edu/bee_lab_mi/153 This Article is brought to you for free and open access by the Bee Lab at DigitalCommons@USU. It has been accepted for inclusion in Mi by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. 4 WWvyvlrWryrXvW-WvWrW^^ I • • •_ ••^«_«).•>.• •.*.« THE UNIVERSITY OF KANSAS SCIENC5;^ULLETIN LIBRARY Vol. 54, No. 4, pp. 75-164 Sept. 21,1990 OCT 23 1990 HARVARD Classification of the Apidae^ (Hymenoptera) BY Charles D. Michener'^ Appendix: Trigona genalis Friese, a Hitherto Unplaced New Guinea Species BY Charles D. Michener and Shoichi F. Sakagami'^ CONTENTS Abstract 76 Introduction 76 Terminology and Materials 77 Analysis of Relationships among Apid Subfamilies 79 Key to the Subfamilies of Apidae 84 Subfamily Meliponinae 84 Description, 84; Larva, 85; Nest, 85; Social Behavior, 85; Distribution, 85 Relationships among Meliponine Genera 85 History, 85; Analysis, 86; Biogeography, 96; Behavior, 97; Labial palpi, 99; Wing venation, 99; Male genitalia, 102; Poison glands, 103; Chromosome numbers, 103; Convergence, 104; Classificatory questions, 104 Fossil Meliponinae 105 Meliponorytes, -
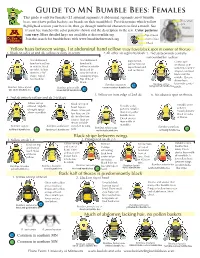
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Recursos Florales Usados Por Dos Especies De Bombus En Un Fragmento De Bosque Subandino (Pamplonita-Colombia)
ARTÍCULO ORIGINAL REVISTA COLOMBIANA DE CIENCIA ANIMAL Rev Colombiana Cienc Anim 2017; 9(1):31-37. Recursos florales usados por dos especies de Bombus en un fragmento de bosque subandino (Pamplonita-Colombia) Floral resources use by two species of Bombus in a subandean forest fragment (Pamplonita-Colombia) 1* 2 3 Mercado-G, Jorge M.Sc, Solano-R, Cristian Biol, Wolfgang R, Hoffmann Biol. 1Universidad de Sucre, Departamento de Biología y Química, Grupo Evolución y Sistemática Tropical. Sincelejo. Colombia. 2IANIGLA, Departamento de Paleontología, CCT CONICET. Mendoza, Argentina. 3Universidad de Pamplona, Facultad de Ciencias Básicas, Grupo de Biocalorimetría. Pamplona, Colombia Keywords: Abstract Pollen; It is present the results of a palynological analysis of two species of the genus palinology; Bombus in vicinity of Pamplonita-Norte of Santander. On feces samples were diet; identified and counted (april and may of 2010) 1585 grains of pollen, within which Norte de Santander; Solanaceae, Asteraceae, Malvaceae and Fabaceae were the more abundat taxa. Hymenoptera, feces. With regard to the diversity, April was the most rich, diverse and dominant month; however in B. pullatus we observed greater wealth and dominance. Finally, it was possible to determine that a large percentage of pollen grains that were identified as Solanaceae correspond to Solanum quitoense. The results of this study make clear the importance of palynology at the time of establishing the diet of these species, in addition to reflect its role of those species as possible provider in ecosistemic services either in wild plant as a crops in Solanaceae case. Palabras Clave: Resumen Polen; Se presentan los resultados de un análisis palinológico en dos especies del palinología; género Bombus en cercanías de Pamplonita (Norte de Santander-Colombia) dieta; durante los meses de abril y mayo de 2010. -

CUTOVERS AS POTENTIAL SUITABLE BEE HABITATS By
CUTOVERS AS POTENTIAL SUITABLE BEE HABITATS by Jasmine R. Pinksen A Thesis submitted in partial fulfillment of the requirements for the degree of Bachelor of Science, Honours Environmental Science, Grenfell Campus Memorial University of Newfoundland April 2017 Corner Brook Newfoundland 2 Grenfell Campus Environmental Science Unit The undersigned certify that they have read, and recommend to the Environmental Science Unit (School of Science and the Environment) for acceptance, a thesis entitled “Cutovers as Potential Suitable Bee Habitats” submitted by Jasmine R. Pinksen in partial fulfillment of the requirements for the degree of Bachelor of Science, Honours. _____________________________ Dr. Julie Sircom (thesis supervisor) _____________________________ Dr. Erin Fraser _____________________________ Dr. Joe Bowden April ____, 2017 3 Acknowledgements Thank you to the valuable research team members who helped with data collection for this project, Geena Arul Jothi, Megan Trotman, Tiffany Fillier, Erika Young, Nicole Walsh, and Abira Mumtaz. Thank you to Barry Elkins with Corner Brook Pulp and Paper Ltd. for financial support in 2015, maps of the logging areas around Corner Brook, NL, and his recommendations on site selection. Special thank you to my supervisor Dr. Julie Sircom, Dr. Erin Fraser and Dr. Joe Bowden for their feedback and support. 4 Abstract Bees are of great importance for pollinating agricultural crops and wild plant communities. Direct human activities such as urbanization, pesticide use, pollution, and introduction of species and pathogens as well as climate change are resulting in habitat loss and fragmentation for bees, causing declines in bee populations worldwide. Lack of suitable habitat is considered to be one of the main factors contributing to these declines. -

The Conservation Management and Ecology of Northeastern North
THE CONSERVATION MANAGEMENT AND ECOLOGY OF NORTHEASTERN NORTH AMERICAN BUMBLE BEES AMANDA LICZNER A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN BIOLOGY YORK UNIVERSITY TORONTO, ONTARIO September 2020 © Amanda Liczner, 2020 ii Abstract Bumble bees (Bombus spp.; Apidae) are among the pollinators most in decline globally with a main cause being habitat loss. Habitat requirements for bumble bees are poorly understood presenting a research gap. The purpose of my dissertation is to characterize the habitat of bumble bees at different spatial scales using: a systematic literature review of bumble bee nesting and overwintering habitat globally (Chapter 1); surveys of local and landcover variables for two at-risk bumble bee species (Bombus terricola, and B. pensylvanicus) in southern Ontario (Chapter 2); identification of conservation priority areas for bumble bee species in Canada (Chapter 3); and an analysis of the methodology for locating bumble bee nests using detection dogs (Chapter 4). The main findings were current literature on bumble bee nesting and overwintering habitat is limited and biased towards the United Kingdom and agricultural habitats (Ch.1). Bumble bees overwinter underground, often on shaded banks or near trees. Nests were mostly underground and found in many landscapes (Ch.1). B. terricola and B. pensylvanicus have distinct habitat characteristics (Ch.2). Landscape predictors explained more variation in the species data than local or floral resources (Ch.2). Among local variables, floral resources were consistently important throughout the season (Ch.2). Most bumble bee conservation priority areas are in western Canada, southern Ontario, southern Quebec and across the Maritimes and are most often located within woody savannas (Ch.3). -

Phylogenetic Analysis of the Corbiculate Bee Tribes Based on 12 Nuclear Protein-Coding Genes (Hymenoptera: Apoidea: Apidae) Atsushi Kawakita, John S
Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae) Atsushi Kawakita, John S. Ascher, Teiji Sota, Makoto Kato, David W. Roubik To cite this version: Atsushi Kawakita, John S. Ascher, Teiji Sota, Makoto Kato, David W. Roubik. Phylogenetic anal- ysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae). Apidologie, Springer Verlag, 2008, 39 (1), pp.163-175. hal-00891935 HAL Id: hal-00891935 https://hal.archives-ouvertes.fr/hal-00891935 Submitted on 1 Jan 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie 39 (2008) 163–175 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2007046 Original article Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae)* Atsushi Kawakita1, John S. Ascher2, Teiji Sota3,MakotoKato 1, David W. Roubik4 1 Graduate School of Human and Environmental Studies, Kyoto University, Kyoto, Japan 2 Division of Invertebrate Zoology, American Museum of Natural History, New York, USA 3 Department of Zoology, Graduate School of Science, Kyoto University, Kyoto, Japan 4 Smithsonian Tropical Research Institute, Balboa, Ancon, Panama Received 2 July 2007 – Revised 3 October 2007 – Accepted 3 October 2007 Abstract – The corbiculate bees comprise four tribes, the advanced eusocial Apini and Meliponini, the primitively eusocial Bombini, and the solitary or communal Euglossini. -

Bumble Bees of CT-Females
Guide to CT Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble bees, most have pollen baskets, no beards on their mandibles). First determine which yellow Three small eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns can vary. More detailed keys are available at discoverlife.org. Top of head Join the search for bumble bees with www.bumbleebeewatch.org Front of face Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee patch. two-spotted bumble bee brown-belted bumble bee 4. 2nd ab entirely yellow and ab 3-6 black 5. No obvious spot on thorax. Yellow on top Black on top of Variable color of head. -

Bumblebee in the UK
There are 24 species of bumblebee in the UK. This field guide contains illustrations and descriptions of the eight most common species. All illustrations 1.5x actual size. There has been a marked decline in the diversity and abundance of wild bees across Europe in recent decades. In the UK, two species of bumblebee have become extinct within the last 80 years, and seven species are listed in the Government’s Biodiversity Action Plan as priorities for conservation. This decline has been largely attributed to habitat destruction and fragmentation, as a result of Queen Worker Male urbanisation and the intensification of agricultural practices. Common The Centre for Agroecology and Food Security is conducting Tree bumblebee (Bombus hypnorum) research to encourage and support bumblebees in food Bumblebees growing areas on allotments and in gardens. Bees are of the United Kingdom Queens, workers and males all have a brown-ginger essential for food security, and are regarded as the most thorax, and a black abdomen with a white tail. This important insect pollinators worldwide. Of the 100 crop species that provide 90% of the world’s food, over 70 are recent arrival from France is now present across most pollinated by bees. of England and Wales, and is thought to be moving northwards. Size: queen 18mm, worker 14mm, male 16mm The Centre for Agroecology and Food Security (CAFS) is a joint initiative between Coventry University and Garden Organic, which brings together social and natural scientists whose collective research expertise in the fields of agriculture and food spans several decades. The Centre conducts critical, rigorous and relevant research which contributes to the development of agricultural and food production practices which are economically sound, socially just and promote long-term protection of natural Queen Worker Male resources. -

Global Trends in Bumble Bee Health
EN65CH11_Cameron ARjats.cls December 18, 2019 20:52 Annual Review of Entomology Global Trends in Bumble Bee Health Sydney A. Cameron1,∗ and Ben M. Sadd2 1Department of Entomology, University of Illinois, Urbana, Illinois 61801, USA; email: [email protected] 2School of Biological Sciences, Illinois State University, Normal, Illinois 61790, USA; email: [email protected] Annu. Rev. Entomol. 2020. 65:209–32 Keywords First published as a Review in Advance on Bombus, pollinator, status, decline, conservation, neonicotinoids, pathogens October 14, 2019 The Annual Review of Entomology is online at Abstract ento.annualreviews.org Bumble bees (Bombus) are unusually important pollinators, with approx- https://doi.org/10.1146/annurev-ento-011118- imately 260 wild species native to all biogeographic regions except sub- 111847 Saharan Africa, Australia, and New Zealand. As they are vitally important in Copyright © 2020 by Annual Reviews. natural ecosystems and to agricultural food production globally, the increase Annu. Rev. Entomol. 2020.65:209-232. Downloaded from www.annualreviews.org All rights reserved in reports of declining distribution and abundance over the past decade ∗ Corresponding author has led to an explosion of interest in bumble bee population decline. We Access provided by University of Illinois - Urbana Champaign on 02/11/20. For personal use only. summarize data on the threat status of wild bumble bee species across bio- geographic regions, underscoring regions lacking assessment data. Focusing on data-rich studies, we also synthesize recent research on potential causes of population declines. There is evidence that habitat loss, changing climate, pathogen transmission, invasion of nonnative species, and pesticides, oper- ating individually and in combination, negatively impact bumble bee health, and that effects may depend on species and locality. -

Portland Field Guide
FIELD GUIDE FRITZ HAEG’S ANIMAL ESTATES REGIONAL MODEL HOMES 5.0 PORTLAND, OREGON 5 . N 0 O P G O RE RTLAND, O ANIMAL ESTATES 5.0 PORTLAND, OREGON DOUGLAS F. COOLEY MEMORIAL ART GALLERY REED COLLEGE 26 AUGUST–5 OCTOBER 2008 FRITZ HAEG 04 INTRODUCTION 10 IN LIVABLE CITIES IS FRITZ HAEG PRESERVATION OF THE WILD MIKE HOUCK 06 BUILD A BETTER SNAG! 14 SNAGS AND LOGS STEPHANIE SNYDER CHARLOTTE CORKRAN ANIMAL CLIENTS 18 CLIENT 5.1 34 CLIENT 5.5 VAUX’S SWIFT NORTHWESTERN GARTER SNAKE CHAETURA VAUXI THAMNOPHIS SIRTALIS TETRATAENIA CONTENTS BOB SALLINGER TIERRA CURRY 2 2 CLIENT 5.2 38 CLIENT 5.6 WHITE-BREASTED NUTHATCH ORANGE-RUMPED BUMBLEBEE SITTA CAROLINENSIS BOMBUS MELANOPYGUS CHARLOTTE CORKRAN CHRISTOPHER MARSHALL 26 CLIENT 5.3 42 CLIENT 5.7 OLIVE-SIDED FLYCATCHER SNAIL-EATING GROUND BEETLE CONTOPUS COOPERI SCAPHINOTUS ANGULATUS CHARLOTTE CORKRAN CHRISTOPHER MARSHALL 30 CLIENT 5.4 SILVER-HAIRED BAT LASIONYCTERIS NOCTIVAGANS CHARLOTTE CORKRAN 46 FIELD NOTES 48 CREDITS CLIENT 5.1 VAUX’S SWIFT CHAETURA VAUXI The ongoing Animal Estates lished between the man-made and the wild. initiative creates dwellings for Animals and their habitats are woven back into our cities, strip malls, garages, offi ce parks, animals that have been displaced freeways, backyards, parking lots, and neigh- by humans. Each edition of borhoods. Animal Estates intends to provide a the project is accompanied by provocative twenty-fi rst-century model for the some combination of events, human-animal relationship that is more intimate, visible, and thoughtful. workshops, exhibitions, videos, printed materials, and a temporary PORTLAND headquarters presenting an ever- In the gallery, the temporary Animal Estates expanding urban wildlife archive.