Thrips Transmitted Viruses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thysanoptera: Thripidae
Preferences of Scirtothrips dorsalis Hood 1919 (Thysanoptera: Thripidae) for different structures of cotton (Gossypium hirsutum L.) plants in the Magdalena warm valley of Colombia Preferencias de Scirtothrips dorsalis Hood 1919 (Thysanoptera: Thripidae) por diferentes estructuras de la planta del algodón (Gossypium hirsutum L.) en el valle cálido del Magdalena Everth Ebratt1*, Andrés Rodríguez2, Buenaventura Monje2, Edgar Varón2, Helena Brochero3, and Arturo Goldarazena4 ABSTRACT RESUMEN Thrips samples were collected from cotton crops in the Andean En la región Andina que comprende el valle cálido del alto region of the Magdalena warm valley, an area represented by the Magdalena representado por los departamentos de Tolima, Colombian departments of Tolima, Huila and Cundinamarca. Huila y Cundinamarca en Colombia se recolectaron muestras Ten cotton plants were randomly selected per hectare in each de trips en cultivos de algodón. En cada predio se selecciona- plot. Five young leaves, five floral buds, five opened flowers ron diez plantas de algodón al azar por hectárea en las cuales and five bolls or fruits were inspected. Immature stages were se inspeccionaron cinco hojas jóvenes o terminales foliares, separated from the adults and a first classification was made cinco botones florales, cinco flores abiertas y cinco cápsulas o according to the present thrips morphotypes, separating the frutos. Los estados inmaduros se separaron de los adultos y se adults of possible S. dorsalis specimens from the others. T- hizo una primera clasificación de acuerdo a los morfotipos de Student and Kruskal-Wallis tests were performed in order trips presentes, separando los adultos de posibles especímenes to find statistical differences between the different evaluated de S. -

Plant Health Карантин Растений
КАРАНТИН РАСТЕНИЙ МАРТ НАУКА И ПРАКТИКА 1| 11| 2015 РУССКО-АНГЛИЙСКИЙ ЖУРНАЛ ТРИПСЫ — КАНДИДАТЫ НА ВКЛЮЧЕНИЕ В ПЕРЕЧЕНЬ КАРАНТИННЫХ ОБЪЕКТОВ ТАМОЖЕННОГО СОЮЗА, ОБНАРУЖИВАЕМЫЕ В ПОДКАРАНТИННОЙ ПРОДУКЦИИ, ПОСТУПАЮЩЕЙ В КАЛИНИНГРАДСКУЮ ОБЛАСТЬ стр. 4 КОЛЬЧАТЫЕ КОКОНОПРЯДЫ РОДА MALACOSOMA — НОВЫЕ ОБЪЕКТЫ КАРАНТИННОГО ПЕРЕЧНЯ РФ стр. 20 ПОИСК МОЛЕКУЛЯРНЫХ МАРКЕРОВ ДЛЯ ИДЕНТИФИКАЦИИ СОРНЫХ РАСТЕНИЙ стр. 32 THRIPS INTERCEPTED IN REGULATED ARTICLES IMPORTED INTO KALININGRAD REGION AS CANDIDATES FOR INCLUSION INTO THE PEST LIST OF THE CUSTOMS UNION page 9 TENT CATERPILLARS OF THE GENUS MALACOSOMA — NEW PESTS IN THE RUSSIAN QUARANTINE LIST page 24 SEARCH FOR MOLECULAR MARKERS FOR IDENTIFICATION OF WEEDS page 36 RUSSIAN-ENGLISH JOURNAL PLANT HEALTH MARCH ISSN 2306-9767 ISSN RESEARCH AND PRACTICE 1| 11| 2015 КАРАНТИН РАСТЕНИЙ 1| 11| 2015 1 СОДЕРЖАНИЕ CONTENT НАУЧНЫЕ ИССЛЕДОВАНИЯ RESEARCH STUDIES «КАРАНТИН РАСТЕНИЙ. НАУКА И ПРАКТИКА» В ОБЛАСТИ КАРАНТИНА РАСТЕНИЙ IN PLANT QUARANTINE ДВУЯЗЫЧНЫЙ НАУЧНЫЙ ЖУРНАЛ №1 (11) 2015 Г. В.И. Рожина, ведущий биолог ФГБУ «Калининградская МВЛ» Viktoria I. Rozhina, Leading Biologist Трипсы — кандидаты на включение в перечень карантинных объектов at the Kaliningrad Interregional Veterinary Laboratory Таможенного союза, обнаруживаемые в подкарантинной продукции, Thrips Intercepted in Regulated Articles Imported into Kaliningrad Region as поступающей в Калининградскую область Candidates for Inclusion into the Pest List of the Customs Union Главный редактор: Санин С.С. — академик РАН, РЕДАКЦИЯ: 4 9 У.Ш. Магомедов, кандидат директор Всероссийского НИИ Волкова Е.М. — заведующая сельскохозяйственных наук, фитопатологии В.Н. Жимерикин, ведущий научный сотрудник ФГБУ «ВНИИКР» Vladimir N. Zhimerikin, FGBU VNIIKR’s Leading Researcher лабораторией сорных растений директор ФГБУ «ВНИИКР» Ю.В. Смирнов, заместитель начальника Yury V. Smirnov, Deputy Head of FGBU VNIIKR’s научно-методического отдела энтомологии ФГБУ «ВНИИКР» Entomological Research and Methodology Department Мартин Уорд — Волков О.Г. -

Molecular Characterization of Tobacco Streak Virus Causing Soybean Necrosis in India
Indian Journal of Biotechnology Vol 7, April 2008, pp 214-217 Molecular characterization of Tobacco streak virus causing soybean necrosis in India N Arun Kumar1,2, M Lakshmi Narasu2, Usha B Zehr1 and K S Ravi1* 1Mahyco Research Center, Jalna-Aurangabad Road, Dawalwadi, Post Box No. 76, Jalna 413 203, India 2Center for Biotechnology, Jawaharlal Nehru Technological University, Kukatpally, Hyderabad 500 072, India Received 13 March 2007; revised 3 May 2007; accepted 13 September 2007 A virus isolate infecting soybean (Glycine max L.) with characteristic symptoms of necrosis was collected from various locations of Maharashtra, India. The virus was detected as Tobacco streak virus (TSV) by direct antigen-coating-enzyme- linked immunosorbent assay (DAC-ELISA) using TSV specific antiserum. ELISA positive TSV soybean isolates upon mechanical transmission onto indicator hosts, viz. Vigna unguiculata cv. C-152, Glycine max and Nicotiana tabacum cv. Xanthi, produced characteristic local necrotic lesions on primary inoculated leaves, followed by systemic infection. Coat protein (CP) gene from a representative soybean isolate (TSV-SB) was amplified using TSV CP specific primers. The amplicon of ~750 bp was cloned and sequenced. The CP gene consists of 717 nucleotides, potential of coding a polypeptide of 238 amino acid residues. The CP gene of TSV-SB isolate shared 98.3 to 99.3% nucleotide and 96.6 to 98.3% amino acid sequence homology with the TSV isolates characterized from India. With TSV-WC (White clover isolate from America, type strain) and TSV-BR (Soybean isolate from Brazil), TSV-SB isolate shared 88.7% and 79.2% amino acid sequence homology, respectively. -

(Thrips Palmi) Associated with Capsicum Chlorosis Virus Infection
RESEARCH ARTICLE Transcriptome-wide responses of adult melon thrips (Thrips palmi) associated with capsicum chlorosis virus infection Shirani M. K. Widana Gamage1¤, Dorith Rotenberg2, Derek J. Schneweis3, Chi-Wei Tsai4, 1 Ralf G. DietzgenID * 1 Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, St. Lucia, Queensland, Australia, 2 Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC, United States of America, 3 Department of Plant Pathology, Kansas State University, a1111111111 Manhattan, KS, United States of America, 4 Department of Entomology, National Taiwan University, Taipei, a1111111111 Taiwan a1111111111 ¤ Current address: Department of Botany, University of Ruhuna, Matara, Sri Lanka a1111111111 * [email protected] a1111111111 Abstract OPEN ACCESS Thrips palmi is a widely distributed major agricultural pest in the tropics and subtropics, causing significant losses in cucurbit and solanaceous crops through feeding damage and Citation: Widana Gamage SMK, Rotenberg D, Schneweis DJ, Tsai C-W, Dietzgen RG (2018) transmission of tospoviruses. Thrips palmi is a vector of capsicum chlorosis virus (CaCV) in Transcriptome-wide responses of adult melon Australia. The present understanding of transmission biology and potential effects of CaCV thrips (Thrips palmi) associated with capsicum on T. palmi is limited. To gain insights into molecular responses to CaCV infection, we per- chlorosis virus infection. PLoS ONE 13(12): formed RNA-Seq to identify thrips transcripts that are differentially-abundant during virus e0208538. https://doi.org/10.1371/journal. pone.0208538 infection of adults. De-novo assembly of the transcriptome generated from whole bodies of T. palmi adults generated 166,445 contigs, of which ~24% contained a predicted open read- Editor: Yulin Gao, Chinese Academy of Agricultural Sciences Institute of Plant Protection, CHINA ing frame. -

Thrips Simplex Distinguishing Features Both Sexes Fully Winged
Thrips simplex Distinguishing features Both sexes fully winged. Body and legs dark brown, tarsi and antennal segment III yellowish brown; fore wings brown, base paler. Antennae 8-segmented; III–IV with forked sense cone. Head with 2 pairs of ocellar setae; pair III small, arising just inside anterior margins of ocellar triangle; postocular setae pairs I & III slightly longer than ocellar setae III, postocular setae pair II minute. Pronotum with 2 pairs of posteroangular setae, outer pair slightly shorter than inner pair; posterior margin with 3–4 Female Head & pronotum pairs of setae. Metanotum reticulate medially, reticles elongate on posterior half, most reticles with faint internal markings; median setae short, arising behind anterior margin; campaniform sensilla absent. Fore wing first vein with about 7 setae on distal half; second vein with about 14 setae. Abdominal tergite II with 3 lateral marginal setae; tergites V–VIII with paired ctenidia, on VIII posteromesad to spiracles; tergite VIII posteromarginal comb of microtrichia complete but slightly irregular; pleurotergites without discal setae, but bearing ciliate microtrichia. Sternite II with 2 pairs of marginal setae, III–VII with 3 pairs; sternite II with 1–2 discal setae, III–VII with about 12 discal Meso & metanota setae in single row. Antenna Antenna Male smaller than female; tergite VIII with no posteromarginal comb; sternites III–VII with large transverse pore plate, discal setae arising laterally. Related species Metanotum The genus Thrips is the second largest genus in the Thysanoptera, and currently includes, worldwide, over 290 species. All members of genus Thrips lack ocellar setae I on the head, and they all have ctenidia on tergite VIII posteromesad to the spiracles. -

Onion Thrips (Thrips Tabaci)
Published by Utah State University Extension and Utah Plant Pest Diagnostic Laboratory ENT-117-08PR March 2008 Onion Thrips (Thrips tabaci) Diane G. Alston, Entomologist • Daniel Drost, Vegetable Specialist What You Should Know • Onion thrips are the most injurious insect pest to onions in Utah. • Immature and adult thrips prefer to feed on young leaves in the inner neck of plants. • Moderate to severe thrips feeding causes reduced bulb size. • Insecticides are a major tool for their control, but thrips are prone to develop resistance. • Long-term, sustainable management of thrips includes crop cultural practices, onion varietal resistance, biological control, and insecticide resistance management. nion thrips, thrips Thrips tabaci (Order Thysanoptera, Thysanoptera OFamily Thripidae), is a key insect pest in most onion production regions of the world. Immature and adult thrips feed with a punch-and-suck behavior that removes leaf chlorophyll causing white to silver patches and Fig. 2. Adult onion thrips have fringed or hairy wings 2 streaks (Fig. 1). Thrips populations increase rapidly under and 7-segmented antennae. hot, arid conditions and can lead to economic crop unsustainable management. Life history characteristics loss. The early bulb enlargement stage of onion growth of onion thrips that enhance their pest status include is the most sensitive to thrips feeding. Insecticides have a short generation time, high reproductive potential, been the primary tactic for their management; however, asexual reproduction by females (parthenogenesis), repeated applications often lead to resistance in the and occurrence of protected, non-feeding life stages. thrips population, suppression of natural enemies, and Recent research has shown that the majority of onion thrips on a plant are in the non-feeding egg stage (60- 75% of total population on an onion plant during late June to August), and thus, not exposed to insecticides and other suppressive tactics. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0071096 A1 Yamada Et Al
US 20100071096A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0071096 A1 Yamada et al. (43) Pub. Date: Mar. 18, 2010 (54) PLANT DISEASE AND INSECT DAMAGE Publication Classification CONTROL COMPOSITION AND PLANT (51) Int. Cl. DISEASE AND INSECT DAMAGE AOIH 5/10 (2006.01) PREVENTION METHOD AOIN 55/10 (2006.01) AOIN 25/26 (2006.01) (75) Inventors: Eiichi Yamada, Chiba (JP): AOIH 5/00 (2006.01) Ryutaro Ezaki, Shiga (JP); AOIH 5/02 (2006.01) Hidenori Daido, Chiba (JP) AOIH 5/08 (2006.01) AOIP3/00 (2006.01) Correspondence Address: BUCHANAN, INGERSOLL & ROONEY PC (52) U.S. Cl. ............................ 800/295: 514/63; 504/100 POST OFFICE BOX 1404 (57) ABSTRACT ALEXANDRIA, VA 22313-1404 (US) The invention provides a plant disease and insect damage control composition including, as active ingredients, dinote (73) Assignee: Mitsui Chemicals, Inc., Minato-ku furan and at least one fungicidal compound; and a plant (JP) disease and insect damage prevention method that includes applying Such a composition to a plant body, Soil, plant seed, (21) Appl. No.: 12/516,966 stored cereal, stored legume, stored fruit, stored vegetable, silage, stored flowering plant, or export/import timber. The (22) PCT Filed: Nov. 22, 2007 invention provides a new plant disease and insect damage (86). PCT No.: PCT/UP2007/072635 control composition and a plant disease and insect damage prevention method with very low toxicity to mammals and S371 (c)(1), fishes, the composition and method showing an effect against (2), (4) Date: May 29, 2009 plural pathogens and pest insects, including emerging resis tant pathogens and resistant pest insect, by application to a (30) Foreign Application Priority Data plant body, soil, plant seed, stored cereal, stored legume, stored fruit, stored vegetable, silage, stored flowering plant, Nov. -

<I>Thrips Palmi</I>
ISPM 27 27 ANNEX 1 ENG DP 1: Thrips palmi Karny INTERNATIONAL STANDARD FOR PHYTOSANITARY MEASURES PHYTOSANITARY FOR STANDARD INTERNATIONAL DIAGNOSTIC PROTOCOLS Produced by the Secretariat of the International Plant Protection Convention (IPPC) This page is intentionally left blank This diagnostic protocol was adopted by the Fifth Session of the Commission on Phytosanitary Measures in March 2010. The annex is a prescriptive part of ISPM 27. ISPM 27 Diagnostic protocols for regulated pests DP 1: Thrips palmi Karny Adopted 2010; published 2016 CONTENTS 1. Pest Information .............................................................................................................................2 2. Taxonomic Information .................................................................................................................3 3. Detection ........................................................................................................................................3 4. Identification ..................................................................................................................................4 4.1 Morphological identification of the adult thrips ..................................................................5 4.1.1 Preparation of thrips for microscopic examination ..............................................................5 4.1.2 Identification of the family Thripidae ..................................................................................5 4.1.3 Identification of the genus Thrips ........................................................................................5 -

Onion Thrips Vs Western Flower Thrips What’S the Difference? Identification, Monitoring & Damage Patterns
Onion thrips vs Western flower thrips What’s the difference? Identification, monitoring & damage patterns Ashley Summerfield1, Sarah Jandricic2, Rose Buitenhuis3 & Cynthia Scott-Dupree1 1. University of Guelph, 2. Ontario Ministry of Food Agriculture & Rural Affairs (OMAFRA), 3. Vineland Research & Innovation Centre Onion thrips (Thrips tabaci) are a well-established insect pest of outdoor crops, but in recent years they have become a notable Same plant, different damage pest of greenhouse floriculture crops. Typical biocontrol-based IPM programs don’t appear to work as well for onion thrips as they do for the dominant species, Western flower thrips (Frankliniella occidentalis). Without a one-size-fits-all management Although they attack many of the same crops, the strategy, knowing which thrips species is in your crop is the first step to keeping your thrips populations in check. damage patterns they cause are different Do I know you? Sticking together OT WFT Species identification requires Monitoring cards are an indispensable tool for keeping an eye on pest populations, close inspection of thrips’ heads but do they work equally well for all thrips species? & shoulders under a microscope Western Efficacy of yellow vs blue cards flower thrips Onion thrips proportions on cards vs crop Onion thrips Three red 80% 80% Onion thrips cause little Western flower thrips feed Grey eyespots eyespots damage to flowers, they feed heavily on flowers between the between the 60% 60% mainly on foliage compound compound eyes 40% 40% eyes Percent of chrysanthemum leaves Long coarse Although smaller, Long coarse 20% 20% damaged by 20 thrips after 2 weeks hairs are only hairs on both onion thrips can cause 60% on the the top and 0% as much (or more!) bottom of the 0% damage to your crop bottom of the Spring Summer Autumn 40% “shoulders” Onion thrips Western than the larger “shoulders” flower thrips Crop Yellow cards (pronotum) (pronotum) 20% western flower thrips. -
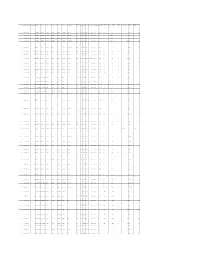
Appendix D: Summary of Accepted ECOTOX Papers
Effect Dur Dur Unit Dur Dur Unit Conc Conc Units Conc Value1 Purity CAS Number Chemical Name Species Number Phylum Class Order Family Genus Species Common Name Group Effect Meas Endpt1 Endpt2 Habitat Plant/Animal Media Orig Orig Preferred Preferred Type Conc Value1 Orig Orig Adjusted 1 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BEH AVO STIM NOAEL terrestrial Animal NONE 30 d 30 d F 2 mg/kg/d 1.96 2 298044 Disulfoton 351 Chordata Actinopterygii Perciformes Anabantidae Anabas testudineus Climbing perch BCM BCM LIPD LOAEL aquatic Animal FW 1 h 4.17E-02 d A 4 mg/L 4 3 298044 Disulfoton 351 Chordata Actinopterygii Perciformes Anabantidae Anabas testudineus Climbing perch BCM BCM PRCO LOAEL aquatic Animal FW 1 h 4.17E-02 d A 4 mg/L 4 4 298044 Disulfoton 351 Chordata Actinopterygii Perciformes Anabantidae Anabas testudineus Climbing perch BCM BCM LIPD LOAEL aquatic Animal FW 1 h 4.17E-02 d A 10.5 mg/L 10.5 5 298044 Disulfoton 4913 Chordata Mammalia Rodentia Muridae Mus musculus House mouse BCM BCM HXBT LOAEL terrestrial Animal NONE 3 d 3 d F 35.1 uM/kg 35.1 6 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BCM BCM GBCM LOAEL terrestrial Animal NONE 24 h 1 d F 0.26 mg/kg 0.234 7 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BCM BCM GBCM IC50 terrestrial Animal NONE 1 wk 7 d F 0.6 ppm 0.6 8 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BCM BCM GBCM NOAEL LOAEL terrestrial Animal NONE -

Abundance of Frankliniella Schultzei (Thysanoptera: Thripidae) in Flowers on Major Vegetable Crops of South Florida Author(S): Garima Kakkar, Dakshina R
Abundance of Frankliniella schultzei (Thysanoptera: Thripidae) in Flowers on Major Vegetable Crops of South Florida Author(s): Garima Kakkar, Dakshina R. Seal, Philip A. Stansly, Oscar E. Liburd and Vivek Kumar Source: Florida Entomologist, 95(2):468-475. 2012. Published By: Florida Entomological Society DOI: http://dx.doi.org/10.1653/024.095.0231 URL: http://www.bioone.org/doi/full/10.1653/024.095.0231 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. 468 Florida Entomologist 95(2) June 2012 ABUNDANCE OF FRANKLINIELLA SCHULTZEI (THYSANOPTERA: THRIPIDAE) IN FLOWERS ON MAJOR VEGETABLE CROPS OF SOUTH FLORIDA GARIMA KAKKAR1,*, DAKSHINA R. SEAL1, PHILIP A. -

Identified Difficulties and Conditions for Field Success of Biocontrol
Identified difficulties and conditions for field success of biocontrol. 4. Socio-economic aspects: market analysis and outlook Bernard Blum, Philippe C. Nicot, Jürgen Köhl, Michelina Ruocco To cite this version: Bernard Blum, Philippe C. Nicot, Jürgen Köhl, Michelina Ruocco. Identified difficulties and conditions for field success of biocontrol. 4. Socio-economic aspects: market analysis and outlook. Classical and augmentative biological control against diseases and pests: critical status analysis and review of factors influencing their success, IOBC - International Organisation for Biological and Integrated Controlof Noxious Animals and Plants, 2011, 978-92-9067-243-2. hal-02809583 HAL Id: hal-02809583 https://hal.inrae.fr/hal-02809583 Submitted on 6 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. WPRS International Organisation for Biological and Integrated Control of Noxious IOBC Animals and Plants: West Palaearctic Regional Section SROP Organisation Internationale de Lutte Biologique et Integrée contre les Animaux et les OILB Plantes Nuisibles: