Guide to Inpatient Quality Indicators: Quality of Care in Hospitals – Volume, Mortality, and Utilization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Diastolic Clicks After the Fontan Procedure for Double
304 Br Heart J 1990;63:304-7 Early diastolic clicks after the Fontan procedure for double inlet left ventricle: anatomical and physiological correlates Br Heart J: first published as 10.1136/hrt.63.5.304 on 1 May 1990. Downloaded from Andrew N Redlngton, Kit-Yee Chan, Julene S Carvalho, Elliot A Shinebourne Abstract inlet ventricle with two atrioventricular M mode echocardiograms and simultan- valves. All had previously undergone a Fontan eous phonocardiograms were recorded type procedure with direct atriopulmonary in four patients with early diastolic anastomosis. In two patients a valved clicks on auscultation. All had double homograft was interposed between the right inlet left ventricle and had undergone atrium and pulmonary artery. Each patient the Fontan procedure with closure of the had the right atrioventricular valve closed by right atrioventricular valve orifice by an either a single or double layer of Dacron cloth artifical patch. The phonocardiogram sewn into the floor of the right atrium. The confirmed a high frequency sound main pulmonary artery was tied off in all. occurring 60-90 ms after aortic valve Cross sectional, M mode, and pulsed wave closure and coinciding with the time of Doppler echocardiograms with simultaneous maximal excursion of the atrioven- electrocardiogram and phonocardiogram were tricular valve patch towards the ven- recorded on a Hewlett-Packard 77020A tricular mass. One patient had coexist- ultrasound system. The phonocardiogram was ing congenital complete heart block. recorded over the point on the chest where the The M mode echocardiogram showed diastolic click was best heart on auscultation, i'reversed") motion of the patch towards usually in the fourth left intercostal space the right atrium during atrial contrac- close to the sternum. -
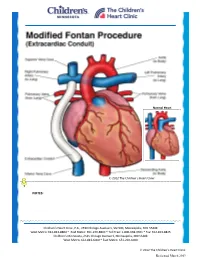
Modified Fontan Procedure: Extracardiac Conduit
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Modified Fontan Procedure: Extracardiac Conduit The Fontan procedure is usually the third procedure done in a series of surgeries to complete palliation of single ventricle patients. This procedure separates the “blue,” deoxygenated blood from the “red,” oxygenated blood circuit. Once the Fontan is done, deoxygenated blood drains passively to the pulmonary arteries, then to the lungs. Blood receives oxygen from the lungs and returns to the heart, where it is actively pumped out to the body. This procedure is usually done between 2-4 years of age. During surgery, the chest is opened through the previous incision, using a median sternotomy. The operation may or may not involve the use of cardiopulmonary bypass (heart-lung machine), depending on the surgical plan. A Gore-tex® tube graft (Gore) is cut to the appropriate length. An incision is made on the pulmonary artery near the area of the existing bidirectional Glenn shunt (cavopulmonary anastomosis) and the tube graft is sutured to the artery. Once that is complete, the inferior vena cava is divided from the atrium. The lower end of the Gore-tex® tube graft (Gore) is then sutured to the inferior vena cava. -

The Physiology of the Fontan Circulation ⁎ Andrew Redington
Progress in Pediatric Cardiology 22 (2006) 179–186 www.elsevier.com/locate/ppedcard The physiology of the Fontan circulation ⁎ Andrew Redington Division of Cardiology, The Department of Pediatrics, The Hospital for Sick Children, University of Toronto School of Medicine, Toronto, Ontario, Canada Available online 8 September 2006 Abstract The Fontan circulation, no matter which of its various iterations, is abnormal in virtually every aspect of its performance. Some of these abnormalities are primarily the result of the procedure itself, and others are secondary to the fundamental disturbances of circulatory performance imposed by the ‘single’ ventricle circulation. The physiology of ventricular, systemic arterial and venopulmonary function will be described in this review. © 2006 Elsevier Ireland Ltd. All rights reserved. Keywords: palliation; Fontan circulation; Cavopulmonary anastomosis; Antriopulmonary anastomosis 1. Introduction has the profound consequences for the systemic ventricle that will discussed below. At the same time as the bidirectional In this personal overview, I will discuss the physiology of the cavopulmonary anastomosis was becoming almost uniformly Fontan circulation. Bob Freedom's contribution to our under- adopted, there was abandonment of the ‘classical’ atriopulmon- standing of complex congenital heart disease has meant that ary anastomosis, in favour of the total cavopulmonary con- many more of these patients are surviving with this physiology nection (TCPC). The ‘lateral wall’ TCPC, popularised by Marc than could have been contemplated 30 years ago. Nonetheless, deLeval [1], was shown experimentally and clinically to be they represent a very difficult group of patients and we continue haemodynamically more efficient [1,2] and set the scene for the to learn more about this unique circulation. -
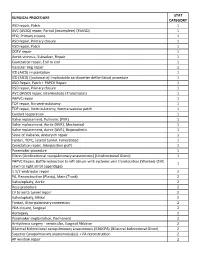
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Nuclear Cardiology
1 XA9847621 Chapter 24 NUCLEAR CARDIOLOGY A. Cuardn Introduction. The assessment of cardiovascular performance with radionuclides dates back to 1927, when Blumgart and Weiss conducted the first clinical studies using a natural bismuth radioisotope 214Bi, in that time known as "Radium C". They injected a solution of this radionuclide into the vein of one arm and detected with a cloud chamber the appearance of its highly penetrating gamma rays in the contralateral arm. Their aim was to study the "velocity of the blood". In these pioneering studies, the mean normal arm-to-arm circulation time proved to be 18 sec., but it was found to be prolonged in patients with heart disease. Subsequently, they were able to calculate the pulmonary circulation time and the pulmonary blood volume by using a forerunner of the Geiger counter with platinum needle electrodes over the right atrium and the left elbow, and to study the effects on them of various heart and lung lesions, thyroid disorders, anaemia, polycythaemia, and drugs. Such classical studies, while appearing crude by today's technology, illustrate that minds and methods were fully prepared to exploit the eventual appearance of the artificial radioisotopes of elements of a more physiological character than bismuth, and laid the foundation for the established techniques of present day nuclear medicine. Although these studies on cardiovascular physiology were the first ever performed in humans with the aid of the radiotracer principle, the cardiologist had to wait for the accumulation of decades of research and development in the fields of radiochemistry, radiopharmacy and instrumentation before being able to capitalize this new approach for the non-invasive investigation of cardiac functions. -

Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Stra
Interstage Monitoring for the Infant with Hypoplastic Left Heart Syndrome What the Direct Care Nurse Needs to Know Jennifer Strawn, BSN, RN, CPN, Children’s Hospital & Medical Center, Omaha, Nebraska Jo Ann Nieves MSN, CPN, ARNP, PNP-BC, FAHA, Miami Children’s Hospital Bronwyn Bartle, DNP, CPNP-AC/PC, Duke Children’s Pediatric and Congenital Heart Center Mary Rummell, MN, RN, CNS, CPNP, FAHA, Oregon Health and Science University Introduction: Children born with hypoplastic left heart syndrome (HLHS) are at high risk for serious morbidity, growth failure and mortality during the time from discharge home after first stage HLHS palliation, until admission for the second stage surgical intervention. To improve outcomes and survival during this time, referred to as the “interstage period”, multiple strategies have been successfully implemented resulting in improved interstage survival. A critical element of interstage care is family education and training. Variations in practice and interstage home surveillance do exist but general guidelines are described below. Definitions: Possible newborn interventions for HLHS Figure 1: First stage surgical palliation procedures for hyoplastic left heart syndrome A. Norwood Procedure B. Norwood Procedure with C. Hybrid Stage I with BT shunt RV to PA shunt (“Sano”) Procedure Surgical: Norwood /Modified Blalock Taussig (BT) shunt procedure – the Norwood aortic reconstruction of the ascending aorta and arch consists of enlarging the ascending aorta by using part of the proximal native main pulmonary artery (PA) with other material (Gore-Tex or homograft material) to connect the PA to the aorta. Pulmonary blood flow is provided by a controlled shunt (BT shunt) usually made from Gore-Tex and connects the right subclavian artery to the right pulmonary artery. -

Long Term Consequences of the Fontan Procedure and How to Manage Them
ACCEPTED MANUSCRIPT Long Term Consequences of the Fontan Procedure and How to Manage Them Authors: W. Aaron Kay, MD1 Tabitha Moe, MD2 Blair Suter, MD3 Andrea Tennancour, NP1 Alice Chan, NP4 Richard A. Krasuski, MD5 Ali N. Zaidi, MD4 1. Indiana University School of Medicine, Krannert Institute of Cardiology, IN 2. University of Arizona School of Medicine, Phoenix, AZ 3. Indiana University School of Medicine, Departments of Medicine and Pediatrics, IN 4. Children’s Hospital at Montefiore, Montefiore Medical Center, Albert Einstein College of Medicine, NY 5. Duke University Health System, Durham, NC *Address reprint requests to: W. Aaron Kay, MD. 1801 N. Senate Blvd Suite 2000, Indianapolis IN. Email: [email protected] Emails of additional authors: Moe TG: [email protected] Suter BC: [email protected] Tennancour AE: [email protected] Chan A: [email protected] Krasuski RA: [email protected] Zaidi AN: [email protected] Disclosures: W. Aaron Kay: Nothing to disclose Tabitha Moe: Nothing to disclose Blair Suter, MD: Nothing to disclose Andrea Tennancour, NP: Nothing to disclose Alice Chan, NP: Nothing to disclose Ali N. Zaidi, MD: Nothing to disclose Richard A. Krasuski, MD: Dr. Krasuski serves a consultant and receives research funding from Actelion Pharmaceuticals.ACCEPTED He also serves as anMANUSCRIPT investigator for Edwards Lifesciences and is an unpaid member of the scientific advisory board for Ventripoint. ___________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Kay, W. A., Moe, T., Suter, B., Tennancour, A., Chan, A., Krasuski, R. A., & Zaidi, A. N. (2018). Long Term Consequences of the Fontan Procedure and How to Manage Them. -

Comparison of Hybrid and Norwood Strategies in Hypoplastic Left Heart Syndrome
SURGERY | REVIEW Comparison of Hybrid and Norwood Strategies in Hypoplastic Left Heart Syndrome Hideyuki Kato, MD, Osami Honjo, MD, PhD, Glen Van Arsdell, MD, Christopher A. Caldarone, MD Division of Cardiovascular Surgery, Hospital for Sick Children, Labatt Family Heart Center Received 12/12/2011, Reviewed 27/12/2011, Accepted 1/1/2012 Key words: Hybrid strategy, Norwood strategy, single ventricle, hypoplastic left heart syndrome DOI: 10.5083/ejcm.20424884.70 ABSTRACT CORRESPONDENCE Current surgical palliation for neonates with single ventricle physiology includes Norwood-based Christopher A. Caldarone, MD, and Hybrid-based surgical strategies. When transplantation is not available, clinicians must choose Professor and Chair, Division of Cardiac Surgery, between these two strategies with distinctly different learning curves and risk profiles. The Norwood University of Toronto, strategy has evolved over several decades while the rising popularity of the Hybrid strategy is a Watson Family Chair in much more recent addition to our therapeutic options. Based upon the premise that avoiding cardio Cardiovascular Science – pulmonary bypass in the neonatal period and deferral of aortic arch reconstruction until a second Labatt Family Heart Center, stage procedure have an important influence on outcomes, the Hybrid strategy has compelling Hospital for Sick Children, theoretical advantages and disadvantages in comparison to the Norwood strategy. The purpose 555 University Avenue, of this review is to summarise the currently available data to support -

Risk Factors for Mortality After the Norwood Procedureq
European Journal of Cardio-thoracic Surgery 22 (2002) 82–89 www.elsevier.com/locate/ejcts Risk factors for mortality after the Norwood procedureq J. William Gaynora,*, William T. Mahleb, Mitchell I. Cohenb, Richard F. Ittenbachc, William M. DeCamplia, James M. Stevend, Susan C. Nicolsond, Thomas L. Spraya Downloaded from https://academic.oup.com/ejcts/article/22/1/82/516394 by guest on 28 September 2021 aDivision of Cardiothoracic Surgery, The Cardiac Center at The Children’s Hospital of Philadelphia, 34th Street and Civic Center Boulevard, Suite 8527, Philadelphia, PA 19104, USA bDivision of Cardiology, The Cardiac Center at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA cDivision of Biostatistics and Epidemiology, The Cardiac Center at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA dDivision of Cardiac Anesthesiology, The Cardiac Center at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA Received 18 September 2001; received in revised form 7 March 2002; accepted 22 March 2002 Abstract Objectives: Recent studies have suggested that survival following the Norwood procedure is influenced by anatomy and is worse for patients with hypoplastic left heart syndrome (HLHS), particularly aortic atresia (AA), as compared to other forms of functional single ventricle and systemic outflow tract obstruction. The current study was undertaken to evaluate our recent experience with the Norwood procedure and to evaluate potential predictors of operative and 1-year mortality. Methods: A retrospective study of risk factors for operative and 1-year mortality in 158 patients undergoing the Norwood procedure between January 1, 1998 and June 30, 2001. Results: HLHS was present in 102 patients (70 with AA) and other forms of functional single ventricle with systemic outflow tract obstruction in the remaining 56. -

MHSUDS Information Notice No.: 16-051
State of California—Health and Human Services Agency Department of Health Care Services JENNIFER KENT EDMUND G. BROWN JR. DIRECTOR GOVERNOR DATE: October 7, 2016 MHSUDS INFORMATION NOTICE N O.: 16-051 TO: COUNTY BEHAVIORAL HEALTH DIRECTORS COUNTY BEHAVIORAL HEALTH DIRECTORS ASSOCIATION OF CALIFORNIA CALIFORNIA COUNCIL OF COMMUNITY BEHAVIORAL HEALTH AGENCIES CALIFORNIA ALLIANCE OF CHILD AND FAMILY SERVICES SUBJECT: IMPLEMENTATION OF THE DIAGNOSTIC AND STATISTICAL MANUAL OF MENTAL DISORDERS (DSM-5), FIFTH EDITION REFERENCES: MHSD INFORMATION NOTICE 13-22 MHSUDS INFORMATION NOTICE 14-040 MHSUDS INFORMATION NOTICE 15-003 MHSUDS INFORMATION NOTICE 15-030 PURPOSE The purpose of this Information Notice is to direct Mental Health Plans (MHPs) to use the American Psychiatric Association (APA) DS M-5 to make diagnostic determinations for the purposes of determining if beneficiaries meet medical necessity criteria for Medi-Cal specialty mental health services (SMHS). This Information Notice updates information previously provided in Mental Health Services Division (MHSD) Information Notice 13-22. BACKGROUND The APA issued the DSM-5 on May 20, 2013. The DSM-5 provides diagnostic criteria and codes as well as corresponding International Classification of Diseases-10 (ICD-10) codes. Since March 2013, the Department of Health Care Services (DHCS) has collaborated with internal and external stakeholders and partners to address implementation issues specific to SMHS related to the DSM-5. Mental Health & Substance Use Disorder Services 1501 Capitol Avenue, MS 4000, P.O. Box 997413 Sacramento, CA 95899-7413 Phone: (916) 440-7800 Fax: (916) 319-8219 Internet Address: www.dhcs.ca.gov MHSUDS INFORMATION NOTICE N O.: 16-051 October 7, 2016 Page 2 DHCS has issued previous MHSUDS Information Notices1 that instruct MHPs to report only ICD-10 codes for claiming and diagnoses reporting purposes and provide ICD-10 procedural and diagnosis crosswalk documents f or SMHS. -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -

Norwood/Batista Operation for a Newborn with Dilated Myopathy of the Left Ventricle
612 Brief communications The Journal of Thoracic and Cardiovascular Surgery September 2000 NORWOOD/BATISTA OPERATION FOR A NEWBORN WITH DILATED MYOPATHY OF THE LEFT VENTRICLE Richard D. Mainwaring, MD, Regina M. Healy, BS, John D. Murphy, MD, and William I. Norwood, MD, PhD, Wilmington, Del Partial left ventriculectomy for dilated cardiomyopathy was contractile function. The variable results that have been first reported by Batista and associates1 in 1996. The rationale reported in the adult literature may reflect patient selection for this procedure is the increase in left ventricular cavity size according to the reversibility or recoverability of the underly- in the absence of compensatory left ventricular wall thickness ing disease process. that is observed in dilated cardiomyopathy. This combination Despite the substantial worldwide experience with the of factors results in an increase in wall stress per unit muscle Batista procedure in adult patients, there is limited experience mass, as predicted by the LaPlace equation. As wall stress with this procedure in children. The current case report increases, mechanical load eventually becomes nonsustain- describes the treatment of a patient in whom the diagnosis of able and contributes to further dilation of the ventricle. Partial dilated cardiomyopathy was made in utero. left ventriculectomy has been advocated as a method of Clinical summary. A female infant was recognized in restoring the balance between cavity size and wall thickness. utero as having a dilated, poorly functioning left ventricle. Fundamental to this concept is the assumption that the left Labor was induced at 36 weeks’ gestation, and she was deliv- ventricular muscle is intrinsically normal or has recoverable ered by normal, spontaneous, vaginal delivery.