Gray-Foot Lancetooth Haplotrema Concavum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

Gray-Foot Lancetooth Haplotrema Concavum ILLINOIS RANGE
gray-foot lancetooth Haplotrema concavum Kingdom: Animalia FEATURES Phylum: Mollusca Three-fourths inch is the maximum shell dimension for this Class: Gastropoda species. Snails have a complex system of organs. The mouth Order: contains a radula, a flexible, ribbonlike structure lined with rows of teeth, used to scrape food. On the head are tentacles. Family: Haplotrematidae Most snails in Illinois have an eye at the tip of each upper ILLINOIS STATUS tentacle. A snail’s shell develops in the egg along with the rest of its body and continues to grow until the snail reaches common, native sexual maturity. The shell is formed by deposits of calcium laid down by the mantle. As the shell grows in its coiled shape, whorls are added. A snail cannot leave its shell. It has a strong muscle inside that is firmly attached to the shell. Snail shells grow in a variety of shapes. Shell shape, number and type of whorls and shell ornamentation, such as ribs or hairs, aid in identification of species. Snail shells may persist long after the snail has died and often can be used to identify species. BEHAVIORS A voracious predatory snail, the gray-foot lancetooth has a specially adapted radula with barbed toothlike projections that enable it to eat the flesh of other snails. Its elongated neck region permits it to extend into the shell of its helpless victim. It is the only predatory land snail in Illinois. Snails need to seek sheltered places to live, eat and rest. They prefer to live in moist areas and are commonly found under logs, loose bark or coarse woody debris, and in leaf litter on the forest floor. -

Mollusques Terrestres De L'archipel De La Guadeloupe, Petites Antilles
Mollusques terrestres de l’archipel de la Guadeloupe, Petites Antilles Rapport d’inventaire 2014-2015 Laurent CHARLES 2015 Mollusques terrestres de l’archipel de la Guadeloupe, Petites Antilles Rapport d’inventaire 2014-2015 Laurent CHARLES1 Ce travail a bénéficié du soutien financier de la Direction de l’Environnement de l’Aménagement et du Logement de la Guadeloupe (DEAL971, arrêté RN-2014-019). 1 Muséum d’Histoire Naturelle, 5 place Bardineau, F-33000 Bordeaux - [email protected] Citation : CHARLES L. 2015. Mollusques terrestres de l’archipel de la Guadeloupe, Petites Antilles. Rapport d’inventaire 2014-2015. DEAL Guadeloupe. 88 p., 11 pl. + annexes. Couverture : Helicina fasciata SOMMAIRE REMERCIEMENTS .................................................................................................................. 2 INTRODUCTION ...................................................................................................................... 3 MATÉRIEL ET MÉTHODES .................................................................................................... 5 RÉSULTATS ............................................................................................................................ 9 Catalogue annoté des espèces ............................................................................................ 9 Espèces citées de manière erronée ................................................................................... 35 Diversité spécifique pour l’archipel .................................................................................... -

The Land and Fresh-Water Mollusks of Puerto Rico
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 70 THE LAND AND FRESH-WATER MOLLUSKS OF PUERTO RICO BY HENRY VAN DER SCHALIE ANN ARBOR UNIVERSITY OF MICHIGAN PRESS AUGUST12, 1948 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 70 THE LAND AND FRESH-WATER MOLLUSKS OF PUERTO RICO BY HENRY VAN DER SCHALIE ANN ARBOR UNIVERSITY OF MICHIGAN PRESS AUGUST12, 1948 CONTENTS INTRODUCTION.............. -... 9 Acknowledgments 10 ILLUSTRATIONS PLATES (Plates I-XITT follo~vpage 128) PLATE Francisco Mariaiio YagBn (front~spiece). I. FIG. 1. Alcadia striata (Lamnrck). FIG. a. Alcadia ILjulnlarsoni (Pfeiffer). FIG. 3. Alcadia ulta (Sowerby). FIa. 4. Helicina pl~asinnella " Sowel.by ' ' Pfeiff er. Fra. 5. Lucidella winosa (Sliuttle~vorth). PIG. 6. Lucidclla umbonuta (5huttlewortl1). FIa. 7. Pad?/enin portoricensis (Pfeiffer). FIG. 8. Ccrutoth.cc~isportoricanus Pilsbry and Vanatta. l17ra. 9. Stoaston~ops~U.C~~O~LC(LPLU $1. lj. 1l:lkcr. F1a. 10. Stoastonlops boriqucni 11. 13. Balter. 11. Fra. 1. Megalomastoma o'oceum (Ginelin). Fra. 2. Megalomasto?na werruculosum Sliuttlcworth. FIG. Licina decttssata (Lamarck). FIG. Licina aguadillensis (Pfeiffer) . FIG. Licina granainosa H. B. Baker. Fro. Chondropoma riisei (Pfeiffer). Fra. Chondropoma blauneri (Shuttleworth). Fra. Cl~ondropomaconseptum (von Martens). FIa. Chondropoma yunquei H. B. Baker. FIG. Chondroporna swifti (Sh~ttleworth). 111. FIG. 1. Pupi8,oma minus Pilsbry. FIG. 2. Pupison~adioscoricola (C. B. Adams). FIG. 3. Bothriopupa tenuidens (C. B. Adams). FIG. 4. Pupoides nitidulus (Pfeiffer) . FIG. 5. Gastrocopta sc.rwilis (Gould). FIG. 6. Gnstrocoptn prllncidn (Pfciffcr). Fra. 7. Guppya pi?~dlachi(Pf~iffer) . Fla. 8. Habroconcts cf. ernsti (Jousseaume). FIa. 9. Hau~aiia~tlinrrsc~rla (I%inney). -

Delaware's Wildlife Species of Greatest Conservation Need
CHAPTER 1 DELAWARE’S WILDLIFE SPECIES OF GREATEST CONSERVATION NEED CHAPTER 1: Delaware’s Wildlife Species of Greatest Conservation Need Contents Introduction ................................................................................................................................................... 7 Regional Context ........................................................................................................................................... 7 Delaware’s Animal Biodiversity .................................................................................................................... 10 State of Knowledge of Delaware’s Species ................................................................................................... 10 Delaware’s Wildlife and SGCN - presented by Taxonomic Group .................................................................. 11 Delaware’s 2015 SGCN Status Rank Tier Definitions................................................................................. 12 TIER 1 .................................................................................................................................................... 13 TIER 2 .................................................................................................................................................... 13 TIER 3 .................................................................................................................................................... 13 Mammals .................................................................................................................................................... -
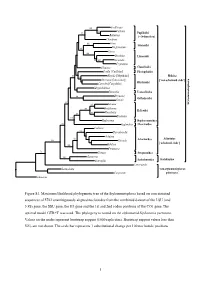
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Biodiversity and Coarse Woody Debris in Southern Forests Proceedings of the Workshop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity
Biodiversity and Coarse woody Debris in Southern Forests Proceedings of the Workshop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity Athens, GA - October 18-20,1993 Biodiversity and Coarse Woody Debris in Southern Forests Proceedings of the Workhop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity Athens, GA October 18-20,1993 Editors: James W. McMinn, USDA Forest Service, Southern Research Station, Forestry Sciences Laboratory, Athens, GA, and D.A. Crossley, Jr., University of Georgia, Athens, GA Sponsored by: U.S. Department of Energy, Savannah River Site, and the USDA Forest Service, Savannah River Forest Station, Biodiversity Program, Aiken, SC Conducted by: USDA Forest Service, Southem Research Station, Asheville, NC, and University of Georgia, Institute of Ecology, Athens, GA Preface James W. McMinn and D. A. Crossley, Jr. Conservation of biodiversity is emerging as a major goal in The effects of CWD on biodiversity depend upon the management of forest ecosystems. The implied harvesting variables, distribution, and dynamics. This objective is the conservation of a full complement of native proceedings addresses the current state of knowledge about species and communities within the forest ecosystem. the influences of CWD on the biodiversity of various Effective implementation of conservation measures will groups of biota. Research priorities are identified for future require a broader knowledge of the dimensions of studies that should provide a basis for the conservation of biodiversity, the contributions of various ecosystem biodiversity when interacting with appropriate management components to those dimensions, and the impact of techniques. management practices. We thank John Blake, USDA Forest Service, Savannah In a workshop held in Athens, GA, October 18-20, 1993, River Forest Station, for encouragement and support we focused on an ecosystem component, coarse woody throughout the workshop process. -

ZM82 615-650 Robinson.Indd
The land Mollusca of Dominica (Lesser Antilles), with notes on some enigmatic or rare species D.G. Robinson, A. Hovestadt, A. Fields & A.S.H. Breure Robinson, D.G., A. Hovestadt, A. Fields & A.S.H. Breure. The land Mollusca of Dominica, Lesser Antilles, with notes on some enigmatic or rare species. Zool. Med. Leiden 83 (13), 9.vii.2009: 615-650, fi gs 1-14, tables 1-2.― ISSN 0024-0672. D.G. Robinson, The Academy of Natural Sciences, 1900 Benjamin Franklin Parkway, Philadelphia, PA 19103, U.S.A. ([email protected]). A. Hovestadt, Dr. Abraham Kuyperlaan 22, NL-3818 JC Amersfoort, The Netherlands (ad.hovestadt@ xs4all.nl). A. Fields, Department of Biological and Chemical Sciences, University of the West Indies, Cave Hill Cam- pus, Barbados (angela.fi [email protected]). A.S.H. Breure, National Museum of Natural History, P.O. Box 9517, NL-2300 RA Leiden, The Netherlands ([email protected]). Key words: Mollusca, Gastropoda, taxonomy, distribution, Dominica. An overview of the land-snail fauna of the Lesser Antillean island of Dominica is given, based on data from literature and four recent surveys. There are 42 taxa listed, of which the following species are recorded for the fi rst time from the island: Allopeas gracile (Hutt on, 1834), A. micra (d’Orbigny, 1835), Beckianum beckianum (L. Pfeiff er, 1846), Bulimulus diaphanus fraterculus (Potiez & Michaud, 1835), De- roceras laeve (Müller, 1774), Sarasinula marginata (Semper, 1885), Streptostele musaecola (Morelet, 1860) and Veronicella sloanii (Cuvier, 1817). The enigmatic Bulimulus stenogyroides Guppy, 1868 is now placed in the genus Naesiotus Albers, 1850. -

A Tentative List of the Land Snails of Georgia, U.S.A. Zachary I
Georgia Journal of Science Volume 77 No. 2 Scholarly Contributions from the Article 8 Membership and Others 2019 A Tentative List of the Land Snails of Georgia, U.S.A. Zachary I. Felix Reinhardt University, [email protected] Michael A. Dubuc Reinhardt University Hassan A. Rana Reinhardt University Follow this and additional works at: https://digitalcommons.gaacademy.org/gjs Part of the Biodiversity Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Felix, Zachary I.; Dubuc, Michael A.; and Rana, Hassan A. (2019) "A Tentative List of the Land Snails of Georgia, U.S.A.," Georgia Journal of Science, Vol. 77, No. 2, Article 8. Available at: https://digitalcommons.gaacademy.org/gjs/vol77/iss2/8 This Research Articles is brought to you for free and open access by Digital Commons @ the Georgia Academy of Science. It has been accepted for inclusion in Georgia Journal of Science by an authorized editor of Digital Commons @ the Georgia Academy of Science. A Tentative List of the Land Snails of Georgia, U.S.A. Acknowledgements We thank Shayla Scott for help with building our database. Thanks to the following individuals for sharing museum data: Adam Baldinger, Clarissa Bey, Rudiger Bieler, Cheryl Bright, Brian Helms, Christine Johnson, Timothy Pearce, Gary Rosenburg, Leslie Skibinski, John Slapcinsky, Jamie Smith, and Lee Taehwan. Timothy Pearce, Kathryn Perez, Amy VanDevender, Wayne VanDevender and John Slapcinsky helped tremendously with sorting out taxonomic issues. Helpful reviews were provided by the VanDevenders as well as John Slapcinsky. This research articles is available in Georgia Journal of Science: https://digitalcommons.gaacademy.org/gjs/vol77/iss2/8 Felix et al.: Land Snails of Georgia A TENTATIVE LIST OF THE LAND SNAILS OF GEORGIA, U.S.A. -

The Morphology of the Reproductive Tracts of the Pacific Northwest Pulmonates
AN ABSTRACT OF THE THESIS OF Clarence Alan Porter for the M.S. in Zoology (Degree) (Major) Date thesis is presented /t ll.l / 5 / /(o L/ U Title The Morphology of the Reproductive Tracts of the Pacific Northwest Pulmonates Abstract approved (Major Professor) The morphology and taxonomic use of the reproductive tracts of some of the Pacific Northwest Pulmonates were studied. The snails examined were, Monadenia fidelis (Gray), Vespericola columbiana (Lea), Allogona townsendiana (Lea), Haplotrema sportella (Gould), and Haplotrema vancouverense (Lea). The length of the reproductive tracts of nine or more individuals of each species were measured in millimeters and these measurements were used as a quantitative means for identifying each species. Each snail not only varies in the length of specific structures, but also was seen to differ from others by the presence or absence and the position of certain structures. Some of the structures used to separate the species were: The muscular collar on the vagina in Haplotrema sportella and Haplotrema vancouverense, along with the size and shape of the talon; the presence or absence of a stimulator, verge, flagellum and epiphallus in Allogona townsendiana and Vespericola columbiana; and the presence of a dart, mucus gland, and mucus ejector in Monadenia fidelis. The use of reproductive tract data in taxonomy appears to provide a more accurate means of identifying species. It does not have the problems that are prevalent in shell characters, nor does it require the skill necessary in preparing and reading radula mounts. Combined with the other taxonomic characters it probably provides a better phylogenetic diagnosis of the various species. -

Snail-Eating Snails of Florida, Gastropoda1 Kurt Auffenberg and Lionel A
EENY251 Snail-Eating Snails of Florida, Gastropoda1 Kurt Auffenberg and Lionel A. Stange2 Introduction Euglandina rosea (Ferussac 1821) In Florida, there are three native and two introduced (Family Spiraxidae)—Rosywolf species of snails, belonging to five different families, that are known to feed on other snails. In addition, several Snail introduced species of the Subulinidae are considered Identification carnivorous, but little is known of their biology, and The shell is large, up to 76 mm in height and 27.5 mm in identification is difficult. diameter, is thick and has prominent growth lines. The shape of the shell is fusiform with a narrow ovate-lunate The best known of the Florida predatory snails is the rosy aperture and a truncated columella. Typically, the shell wolfsnail, Euglandina rosea (Férussac 1821), which was color is brownish-pink. Adults measure 7–10 cm in length. exported to Hawaii and other areas (Mead 1961) in vain attempts to control the giant African snail, (Achatina fulica Bowdich 1821). A Mediterranean snail, the decollate snail, Rumina decollata (Linnaeus 1758), is much heralded today (Fisher et al. 1980) in California as an effective biological control agent of the brown garden snail, Cornu aspersum (Müller 1774). Relatively little is known of the other three species of snail-eating snails, two of which are less than 10 mm long. All of these Florida predaceous snails are easy to identify and the following account summarizes what is known of their distributions, identification, and habits. Figure 1. The rosy wolfsnail, Euglandina rosea (Férussac 1821). Credits: Lyle, J. Buss, UF/IFAS Distribution In the United States: Alabama, Florida, Georgia, Hawaii, Louisiana, Mississippi, South Carolina, and southeastern Texas. -

In Eastern Ontario, Canada
14 4 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 14 (4): 579–583 https://doi.org/10.15560/14.4.579 Guppya sterkii (Dall, 1888) (Gastropoda, Eupulmonata, Euconulidae) in eastern Ontario, Canada Robert G. Forsyth,1 Annegret Nicolai2 1 Research Associate, New Brunswick Museum, 277 Douglas Avenue, Saint John, NB, Canada E2K 1E5. 2 UMR CNRS 6553 EcoBio, Station Biologique Paimpont, Université Rennes 1, 35380 Paimpont, France. Corresponding author: Robert G. Forsyth, [email protected] Abstract A seldom-collected terrestrial snail, Guppya sterkii (Dall, 1888), is recorded for the first time from an older-growth hardwood forest in rural Ottawa, eastern Ontario. This represents a range extension of roughly 175 km north-east of the nearest previously known occurrence. Its conservation status and possible threats are briefly discussed. Key words Land snail; Mollusca; range extension; leaf litter; earthworms; Mixedwood Plains ecozone. Academic editor: Leonardo Santos de Souza | Received 16 April 2018 | Accepted 19 June 2018 | Published 13 July 2018 Citation: Forsyth RG, Nicolai AN (2018) Guppya sterkii (Dall, 1888) (Gastropoda, Eupulmonata, Euconulidae) in eastern Ontario, Canada. Check List 14 (4) 579–583. https://doi.org/10.15560/14.4.579 Introduction previously unrecorded species in the region (e.g., Forsyth and Oldham 2014) within the remnants of old-growth Historically, the molluscs of the Ottawa region were hardwood forests in this region. rather well enumerated by early members of the Ottawa Field-Naturalists’ Club, as well as later researchers, The minute terrestrial snail Guppya sterkii (Dall, who reported on highlights from collecting activities, 1888) (Gastropoda, Eupulmonata, Euconulidae) went prepared lists, and issued updates (e.g., Heron 1880, unrecorded from the Canadian fauna until the extensive Latchford 1887, La Rocque 193l).