Terrestrial Gastropods As Focal Species for Monitoring the Ecological
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interior Columbia Basin Mollusk Species of Special Concern
Deixis l-4 consultants INTERIOR COLUMl3lA BASIN MOLLUSK SPECIES OF SPECIAL CONCERN cryptomasfix magnidenfata (Pilsbly, 1940), x7.5 FINAL REPORT Contract #43-OEOO-4-9112 Prepared for: INTERIOR COLUMBIA BASIN ECOSYSTEM MANAGEMENT PROJECT 112 East Poplar Street Walla Walla, WA 99362 TERRENCE J. FREST EDWARD J. JOHANNES January 15, 1995 2517 NE 65th Street Seattle, WA 98115-7125 ‘(206) 527-6764 INTERIOR COLUMBIA BASIN MOLLUSK SPECIES OF SPECIAL CONCERN Terrence J. Frest & Edward J. Johannes Deixis Consultants 2517 NE 65th Street Seattle, WA 98115-7125 (206) 527-6764 January 15,1995 i Each shell, each crawling insect holds a rank important in the plan of Him who framed This scale of beings; holds a rank, which lost Would break the chain and leave behind a gap Which Nature’s self wcuid rue. -Stiiiingfieet, quoted in Tryon (1882) The fast word in ignorance is the man who says of an animal or plant: “what good is it?” If the land mechanism as a whole is good, then every part is good, whether we understand it or not. if the biota in the course of eons has built something we like but do not understand, then who but a fool would discard seemingly useless parts? To keep every cog and wheel is the first rule of intelligent tinkering. -Aido Leopold Put the information you have uncovered to beneficial use. -Anonymous: fortune cookie from China Garden restaurant, Seattle, WA in this “business first” society that we have developed (and that we maintain), the promulgators and pragmatic apologists who favor a “single crop” approach, to enable a continuous “harvest” from the natural system that we have decimated in the name of profits, jobs, etc., are fairfy easy to find. -

Euconulus Alderi Gray a Land Snail
Euconulus alderi, Page 1 Euconulus alderi Gray A land snail State Distribution Photo by Matthew Barthel and Jeffery C. Nekola Best Survey Period Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Status: State listed as Special Concern are smaller, have a more shiny luster, and a darker shell color. Also, the microscopic spiral lines on the base of Global and state ranks: G3Q/S2 the shell are stronger than the radial striations. This is reversed in E. fulvus (Nekola 1998). For more Family: Helicarionidae information on identifying land snails, see Burch and Jung (1988) pages 155-158 or Burch and Pearce (1990) Synonyms: none pages 211-218. Total range: The global range of Euconulus alderi Best survey time: Surveys for E. alderi are best includes Ireland, Sweden, the United Kingdom, and the performed after rain, when the soil and vegetation are United States. Within the U.S. it has been found in moist. During dry periods, a survey site can appear Iowa, Maine, Massachusetts, Michigan, Minnesota, and completely devoid of snails, while after a rain the same Wisconsin (Frest 1990, NatureServe 2007, Nekola site can be found to contain numerous individuals. 1998). This species was not known from North Temperatures should be warm enough that the ground is America until 1986 when it was discovered in Iowa and not frozen and there is no snow. Dry, hot periods during Wisconsin (Frest 1990, Nekola 1998). mid-summer should be avoided. The best time of day to survey is often in early morning when conditions are State distribution: E. -

2010 Animal Species of Concern
MONTANA NATURAL HERITAGE PROGRAM Animal Species of Concern Species List Last Updated 08/05/2010 219 Species of Concern 86 Potential Species of Concern All Records (no filtering) A program of the University of Montana and Natural Resource Information Systems, Montana State Library Introduction The Montana Natural Heritage Program (MTNHP) serves as the state's information source for animals, plants, and plant communities with a focus on species and communities that are rare, threatened, and/or have declining trends and as a result are at risk or potentially at risk of extirpation in Montana. This report on Montana Animal Species of Concern is produced jointly by the Montana Natural Heritage Program (MTNHP) and Montana Department of Fish, Wildlife, and Parks (MFWP). Montana Animal Species of Concern are native Montana animals that are considered to be "at risk" due to declining population trends, threats to their habitats, and/or restricted distribution. Also included in this report are Potential Animal Species of Concern -- animals for which current, often limited, information suggests potential vulnerability or for which additional data are needed before an accurate status assessment can be made. Over the last 200 years, 5 species with historic breeding ranges in Montana have been extirpated from the state; Woodland Caribou (Rangifer tarandus), Greater Prairie-Chicken (Tympanuchus cupido), Passenger Pigeon (Ectopistes migratorius), Pilose Crayfish (Pacifastacus gambelii), and Rocky Mountain Locust (Melanoplus spretus). Designation as a Montana Animal Species of Concern or Potential Animal Species of Concern is not a statutory or regulatory classification. Instead, these designations provide a basis for resource managers and decision-makers to make proactive decisions regarding species conservation and data collection priorities in order to avoid additional extirpations. -

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

Gray-Foot Lancetooth Haplotrema Concavum ILLINOIS RANGE
gray-foot lancetooth Haplotrema concavum Kingdom: Animalia FEATURES Phylum: Mollusca Three-fourths inch is the maximum shell dimension for this Class: Gastropoda species. Snails have a complex system of organs. The mouth Order: contains a radula, a flexible, ribbonlike structure lined with rows of teeth, used to scrape food. On the head are tentacles. Family: Haplotrematidae Most snails in Illinois have an eye at the tip of each upper ILLINOIS STATUS tentacle. A snail’s shell develops in the egg along with the rest of its body and continues to grow until the snail reaches common, native sexual maturity. The shell is formed by deposits of calcium laid down by the mantle. As the shell grows in its coiled shape, whorls are added. A snail cannot leave its shell. It has a strong muscle inside that is firmly attached to the shell. Snail shells grow in a variety of shapes. Shell shape, number and type of whorls and shell ornamentation, such as ribs or hairs, aid in identification of species. Snail shells may persist long after the snail has died and often can be used to identify species. BEHAVIORS A voracious predatory snail, the gray-foot lancetooth has a specially adapted radula with barbed toothlike projections that enable it to eat the flesh of other snails. Its elongated neck region permits it to extend into the shell of its helpless victim. It is the only predatory land snail in Illinois. Snails need to seek sheltered places to live, eat and rest. They prefer to live in moist areas and are commonly found under logs, loose bark or coarse woody debris, and in leaf litter on the forest floor. -

Integrating Life History Traits Into Predictive Phylogeography
Received: 20 August 2018 | Revised: 4 January 2019 | Accepted: 16 January 2019 DOI: 10.1111/mec.15029 ORIGINAL ARTICLE Integrating life history traits into predictive phylogeography Jack Sullivan1,2* | Megan L. Smith3* | Anahí Espíndola1,4 | Megan Ruffley1,2 | Andrew Rankin1,2 | David Tank1,2 | Bryan Carstens3 1Department of Biological Sciences, University of Idaho, Moscow, Abstract Idaho Predictive phylogeography seeks to aggregate genetic, environmental and taxonomic 2 Institute for Bioinformatics and data from multiple species in order to make predictions about unsampled taxa using Evolutionary Studies, University of Idaho, Moscow, Idaho machine‐learning techniques such as Random Forests. To date, organismal trait data 3Department of Ecology, Evolution and have infrequently been incorporated into predictive frameworks due to difficulties Organismal Biology, The Ohio State University, Columbus, Ohio inherent to the scoring of trait data across a taxonomically broad set of taxa. We re‐ 4Department of Entomology, University of fine predictive frameworks from two North American systems, the inland temperate Maryland, College Park, Maryland rainforests of the Pacific Northwest and the Southwestern Arid Lands (SWAL), by Correspondence incorporating a number of organismal trait variables. Our results indicate that incor‐ Jack Sullivan, Department of Biological porating life history traits as predictor variables improves the performance of the Sciences, University of Idaho, Moscow, ID. Email: [email protected] supervised machine‐learning approach to predictive phylogeography, especially for Funding information the SWAL system, in which predictions made from only taxonomic and climate vari‐ National Science Foundation, Grant/Award ables meets only moderate success. In particular, traits related to reproduction (e.g., Numbers: DEB 14575199, DEB 1457726, DG‐1343012; NSF GRFP; Ohio State reproductive mode; clutch size) and trophic level appear to be particularly informa‐ University; Institute for Bioinformatics and tive to the predictive framework. -

Land Snails and Slugs (Gastropoda: Caenogastropoda and Pulmonata) of Two National Parks Along the Potomac River Near Washington, District of Columbia
Banisteria, Number 43, pages 3-20 © 2014 Virginia Natural History Society Land Snails and Slugs (Gastropoda: Caenogastropoda and Pulmonata) of Two National Parks along the Potomac River near Washington, District of Columbia Brent W. Steury U.S. National Park Service 700 George Washington Memorial Parkway Turkey Run Park Headquarters McLean, Virginia 22101 Timothy A. Pearce Carnegie Museum of Natural History 4400 Forbes Avenue Pittsburgh, Pennsylvania 15213-4080 ABSTRACT The land snails and slugs (Gastropoda: Caenogastropoda and Pulmonata) of two national parks along the Potomac River in Washington DC, Maryland, and Virginia were surveyed in 2010 and 2011. A total of 64 species was documented accounting for 60 new county or District records. Paralaoma servilis (Shuttleworth) and Zonitoides nitidus (Müller) are recorded for the first time from Virginia and Euconulus polygyratus (Pilsbry) is confirmed from the state. Previously unreported growth forms of Punctum smithi Morrison and Stenotrema barbatum (Clapp) are described. Key words: District of Columbia, Euconulus polygyratus, Gastropoda, land snails, Maryland, national park, Paralaoma servilis, Punctum smithi, Stenotrema barbatum, Virginia, Zonitoides nitidus. INTRODUCTION Although county-level distributions of native land gastropods have been published for the eastern United Land snails and slugs (Gastropoda: Caeno- States (Hubricht, 1985), and for the District of gastropoda and Pulmonata) represent a large portion of Columbia and Maryland (Grimm, 1971a), and Virginia the terrestrial invertebrate fauna with estimates ranging (Beetle, 1973), no published records exist specific to between 30,000 and 35,000 species worldwide (Solem, the areas inventoried during this study, which covered 1984), including at least 523 native taxa in the eastern select national park sites along the Potomac River in United States (Hubricht, 1985). -
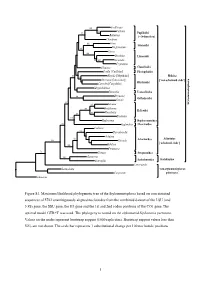
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Terrestrial Mollusk Surveys on Region 1 USFS Lands
Terrestrial Mollusk Surveys on Region 1 USFS Lands Paul Hendricks, Bryce Maxell, Susan Lenard, Coburn Currier, and Ryan Kilacky Montana Natural Heritage Program http://nhp.nris.state.mt.us Globally Rare Land Snails Present on R1 Forests • Selway Forestsnail (Allogona lombardii) (ID) G1 • Dry Land Forestsnail (Allogona ptychophora solida) (ID)? G5T2T3 • Nimapuna Tigersnail (Anguispira nimapuna) (ID) G1 • Chrome Ambershell (Catinella rehderi) (MT, ID?) G1G2Q* • Salmon Oregonian (Cryptomastix harfordiana) (ID)? G3G4 • Mission Creek Oregonian (Cryptomastix magnidentata) (ID)? G1 • Oregonian (Cryptomastix mullani blandi) (ID)? G4T1 • River of No Return Oregonian (Cryptomastix mullani clappi) (ID) G4T1 • Kingston Oregonian (Cryptomastix sanburni) (ID)? G1 SUMMARY • Lake Disc (Discus brunsoni) (MT)? G1 • Marbled Disc (Discus marmorensis) (ID) G1G3 • 31 Species G1-G3 so USFS SOC • Striate Disc (Discus shimekii) (MT, ID?) G5 • 2 Species G5, but S1-S3 so USFS SOI • Salmon Coil (Helicodiscus salmonaceus) (ID) G1G2 • Alpine Mountainsnail (Oreohelix alpina) (MT) G1 • Bitterroot Mountainsnail (Oreohelix amariradix) (MT) G1G2 • Keeled Mountainsnail (Oreohelix carinifera) (MT) G1 • Carinate Mountainsnail (Oreohelix elrodi) (MT) G1 • Seven Devils Mountainsnail (Oreohelix hammeri) (ID) G1 • A Land Snail (Hells Canyon) (Oreohelix idahoensis baileyi) (ID) G1G2T1 • Costate Mountainsnail (Oreohelix idahoensis idahoensis) (ID)? G1G2T1T2 • Deep Slide Mountainsnail (Oreohelix intersum) (ID)? G1 • Boulder Pile Mountainsnail (Oreohelix jugalis) (ID)? G1 • Berry’s -

The Morphology of the Reproductive Tracts of the Pacific Northwest Pulmonates
AN ABSTRACT OF THE THESIS OF Clarence Alan Porter for the M.S. in Zoology (Degree) (Major) Date thesis is presented /t ll.l / 5 / /(o L/ U Title The Morphology of the Reproductive Tracts of the Pacific Northwest Pulmonates Abstract approved (Major Professor) The morphology and taxonomic use of the reproductive tracts of some of the Pacific Northwest Pulmonates were studied. The snails examined were, Monadenia fidelis (Gray), Vespericola columbiana (Lea), Allogona townsendiana (Lea), Haplotrema sportella (Gould), and Haplotrema vancouverense (Lea). The length of the reproductive tracts of nine or more individuals of each species were measured in millimeters and these measurements were used as a quantitative means for identifying each species. Each snail not only varies in the length of specific structures, but also was seen to differ from others by the presence or absence and the position of certain structures. Some of the structures used to separate the species were: The muscular collar on the vagina in Haplotrema sportella and Haplotrema vancouverense, along with the size and shape of the talon; the presence or absence of a stimulator, verge, flagellum and epiphallus in Allogona townsendiana and Vespericola columbiana; and the presence of a dart, mucus gland, and mucus ejector in Monadenia fidelis. The use of reproductive tract data in taxonomy appears to provide a more accurate means of identifying species. It does not have the problems that are prevalent in shell characters, nor does it require the skill necessary in preparing and reading radula mounts. Combined with the other taxonomic characters it probably provides a better phylogenetic diagnosis of the various species. -

Terrestrial Molluscs of the Tsyr-Pripyat Area in Volyn (Northern Ukraine): the First Findings of the Threatened Snail Vertigo Moulinsiana in Mainland Ukraine
Vestnik zoologii, 51(3): 251–258, 2017 DOI 10.1515/vzoo-2017-0031 UDC 594.38(477.82) TERRESTRIAL MOLLUSCS OF THE TSYR-PRIPYAT AREA IN VOLYN (NORTHERN UKRAINE): THE FIRST FINDINGS OF THE THREATENED SNAIL VERTIGO MOULINSIANA IN MAINLAND UKRAINE I. Balashov1, M. Yarotskaya2, J. Filatova3, I. Starichenko3, V. Kovalov3 1Schmalhausen Institute of Zoology, NAS of Ukraine, vul. B. Khmelnytskogo, 15, Kyiv, 01030 Ukraine E-mail: [email protected] 2NatureCenter “Pathfi nders”, Kharkiv, 61091, PO Box 7384, Ukraine E-mail: [email protected] 3V. N. Karazin Kharkiv National University, Svobody Sq., 4, Kharkiv, 61022 Ukraine Terrestrial Molluscs of the Tsyr-Pripyat Area in Volyn (Northern Ukraine): the First Findings of the Th reatened Snail Vertigo moulinsiana in Mainland Ukraine. Balashov, I., Yarotskaya, M., Filatova, J., Starichenko, I., Kovalov, V. — 26 species of terrestrial molluscs were found in the studied area, including the rare and globally threatened Vertigo moulinsiana that is listed in “Habitats Directive” of the EU and in numerous red lists. Until now it was known in Ukraine only by one population in the Crimea that became extinct in 2014. Its conservation status, taking threats into account, is considered to be “Critically Endangered” on the national level in Ukraine. Th e characteristics of the phytocenoses to which it is restricted and the associated molluscan faunas are discussed. Key words: Mollusca, Gastropoda, Stylommatophora, fens, conservation. Introduction Several species of terrestrial molluscs that inhabit European fens are considered to be of high conserva- tion priority. First of all, it refers to the 4 species of the genus Vertigo listed in Annex II of European Unionʹs “Habitats Directive” and numerous red lists: V.moulinsiana (Dupuy, 1849), V. -

Snail-Eating Snails of Florida, Gastropoda1 Kurt Auffenberg and Lionel A
EENY251 Snail-Eating Snails of Florida, Gastropoda1 Kurt Auffenberg and Lionel A. Stange2 Introduction Euglandina rosea (Ferussac 1821) In Florida, there are three native and two introduced (Family Spiraxidae)—Rosywolf species of snails, belonging to five different families, that are known to feed on other snails. In addition, several Snail introduced species of the Subulinidae are considered Identification carnivorous, but little is known of their biology, and The shell is large, up to 76 mm in height and 27.5 mm in identification is difficult. diameter, is thick and has prominent growth lines. The shape of the shell is fusiform with a narrow ovate-lunate The best known of the Florida predatory snails is the rosy aperture and a truncated columella. Typically, the shell wolfsnail, Euglandina rosea (Férussac 1821), which was color is brownish-pink. Adults measure 7–10 cm in length. exported to Hawaii and other areas (Mead 1961) in vain attempts to control the giant African snail, (Achatina fulica Bowdich 1821). A Mediterranean snail, the decollate snail, Rumina decollata (Linnaeus 1758), is much heralded today (Fisher et al. 1980) in California as an effective biological control agent of the brown garden snail, Cornu aspersum (Müller 1774). Relatively little is known of the other three species of snail-eating snails, two of which are less than 10 mm long. All of these Florida predaceous snails are easy to identify and the following account summarizes what is known of their distributions, identification, and habits. Figure 1. The rosy wolfsnail, Euglandina rosea (Férussac 1821). Credits: Lyle, J. Buss, UF/IFAS Distribution In the United States: Alabama, Florida, Georgia, Hawaii, Louisiana, Mississippi, South Carolina, and southeastern Texas.