Estimation of Transformed Reaction Gibbs Energy for Thermodynamically Constraining Metabolic Reaction Networks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Large-Area Coating of Previtamin D3 Based on Roll-To-Roll Processing
coatings Article Large-Area Coating of Previtamin D3 Based on Roll-to-Roll Processing 1, 1, 1 1 1 Janghoon Park y, Yoonki Min y, Jongsu Lee , Hakyung Jeong , Youngwook Noh , Kee-Hyun Shin 2 and Dongjin Lee 2,* 1 Department of Mechanical Design and Production Engineering, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Korea; [email protected] (J.P.); [email protected] (Y.M.); [email protected] (J.L.); [email protected] (H.J.); [email protected] (Y.N.) 2 School of Mechanical Engineering, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-450-0452 These authors contributed equally to this work. y Received: 22 August 2019; Accepted: 9 September 2019; Published: 11 September 2019 Abstract: We propose a roll-to-roll process for vitamin D3 patch production. A solution of 7-dehydrocholesterol is applied to a plastic film by roll-to-roll slot-die coating and dried by a far-infrared lamp. Upon exposure to ultraviolet B irradiation, these films are converted to previtamin D3 films. After heat-treating the previtamin D3 film, high-performance liquid chromatography measurements are performed using commercial vitamin D3 as a standard sample. The results confirm that vitamin D3 can be produced by large-area coating and post-treatment processes. Specifically, 3.16 0.746 mg of vitamin D is obtained through ultraviolet B irradiation and heat-treatment of ± 3 24.8 1.44 mg of coated 7-dehydrocholesterol. ± Keywords: slot-die coating; roll-to-roll (R2R); vitamin D3; ultraviolet B (UVB); high-performance liquid chromatography (HPLC) 1. -

Method Development and Validation of Vitamin D2 and Vitamin D3 Using Mass Spectrometry
Method Development and Validation of Vitamin D2 and Vitamin D3 Using Mass Spectrometry Devon Victoria Riley A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science University of Washington 2016 Committee: Andrew Hoofnagle Geoffrey Baird Dina Greene Program Authorized to Offer Degree: Laboratory Medicine ©Copyright 2016 Devon V. Riley ii University of Washington Abstract Method Development and Validation of Vitamin D2 and Vitamin D3 Using Mass Spectrometry Devon V. Riley Chair of the Supervisory Committee: Associate Professor Andrew Hoofnagle, MD, PhD Vitamin D has long been known to maintain bone health by regulating calcium and phosphorous homeostasis. In recent years, scientists have discovered additional physiological roles for vitamin D. The complex interaction between the active vitamin D hormone and its metabolic precursors continues to be a rich area of research. Fundamental to this research is the availability of accurate and precise assays. Few published assays for vitamins D2 and D3 have contained sufficient details on method validation or performance characteristics. The liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay developed for this thesis has undergone a rigorous validation and proven to yield a sensitive and specific method that exceeds the capabilities of all previously published methods. Developing and validating a novel assay is often complicated by the lack of established acceptability standards. This thesis explores this challenge, specifically for establishing meaningful interpretations and qualification standards of the lower limit of the measuring interval. Altogether, future research focused on vitamins D2, D3 and the Vitamin D pathway can benefit from this robust LC-MS/MS assay and the associated quality parameters outlined in this thesis. -
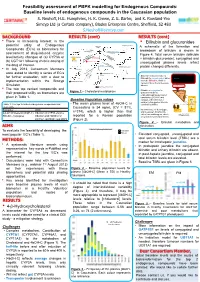
Baseline Levels of Endogenous Compounds in the Caucasian Population S
Feasibility assessment of PBPK modelling for Endogenous Compounds: Baseline levels of endogenous compounds in the Caucasian population S. Neuhoff, H.E. Humphries, H. K. Crewe, Z. E. Barter, and K. Rowland-Yeo Simcyp Ltd (a Certara company), Blades Enterprise Centre, Sheffield, S2 4SU [email protected] BACKGROUND RESULTS (cont) RESULTS (cont) • There is increasing interest in the • Bilirubin and glucuronides 27-Hydroxycholesterol potential utility of Endogenous Cholesterol 1g/day A schematic of the formation and Compounds (EC’s) as biomarkers for CYP7A1 (liver) 500 mg/day CYP27A1 (many tissues) breakdown of bilirubin is shown in assessment of drug-induced enzyme 19 mg/day Figure 4. Total serum bilirubin (bilirubin 7α-Hydroxycholesterol level/activity changes of (a) CYP3A or CYP3A4 + bilirubin glucuronide), conjugated and Bile Acids (b) UGT1A1 following chronic dosing of 4-Hydroxycholesterol unconjugated plasma levels reflect the drug of interest. CYP27A1 (liver and extrahepatic) 24S-Hydroxycholesterol protein changes differently. • In July 2013, Consortium Members Pregnenolone were asked to identify a series of EC’s 4,27-Dihydroxycholesterol Macrophage CYP27A1 • Biliverdin reductase reduces Heme Hemeoxygenase for further evaluation, with a view to 4,24-Dihydroxycholesterol Biliverdin to unconjugated, water- Biliverdin 4,7α-Dihydroxycholesterol insoluble Bilirubin, which is Biliverdin reductase implementation within the Simcyp Bilirubin carried in blood bound to serum Unconjugated bilirubin Simulator. albumin complexed with albumin 3 -

Draft Vitamin D and Health Report
Draft Vitamin D and Health report Scientific consultation: 22 July to 23 September 2015 Contents Page 1. Introduction 3 2. Biology and metabolism 5 3. Photobiology of vitamin D 16 4. Measuring vitamin D exposure (from diet and sunlight) 24 5. Relationship between vitamin D exposure and serum (25(OH)D concentration 29 6. Vitamin D and health outcomes 35 Musculoskeletal outcomes 37 Rickets 39 Osteomalacia 41 Pregnancy and lactation 42 Infants (up to 12 months) 44 Children (1-3y) 45 Children (4-8y) 45 Adults < 50 years 47 Adults > 50 years 50 Conclusions – vitamin D & musculoskeletal health outcomes 59 Non-musculoskeletal health outcomes 61 Pregnancy & lactation – non-musculoskeletal health outcomes 61 Cancers 67 Cardiovascular disease & hypertension 70 All-cause mortality 74 Autoimmune disease 76 Infectious disease 81 Neuropsychological functioning 85 Oral health 88 Age-related macular degeneration 90 Conclusions – non-musculoskeletal health outcomes 92 Selection of health outcomes to inform the setting of DRVs for vitamin D 93 7. Potential adverse effects of high vitamin D intakes/high serum 25(OH)D 95 concentration 8. Dietary vitamin D intakes and serum/plasma 25(OH)D concentrations of the UK 102 population 9. Review of DRVs 109 10. Overall summary & conclusions 121 11. Recommendations 130 12. References 131 2 1. Introduction Background 1. Vitamin D is synthesised in the skin by the action of sunlight. Skin synthesis is the main source of vitamin D for most people; dietary sources are essential when exposure to sunlight containing the appropriate wavelength is limited. The Committee on Medical Aspects of Food and Nutrition Policy (COMA), which set Dietary Reference Values (DRVs) for vitamin D in 1991 (DH1, 1991), did not set a Reference Nutrient Intake (RNI2) for groups in the population considered to receive adequate sunlight exposure. -

Vitamin D and Cancer
WORLD HEALTH ORGANIZATION INTERNATIONAL AGENCY FOR RESEARCH ON CANCER Vitamin D and Cancer IARC 2008 WORLD HEALTH ORGANIZATION INTERNATIONAL AGENCY FOR RESEARCH ON CANCER IARC Working Group Reports Volume 5 Vitamin D and Cancer - i - Vitamin D and Cancer Published by the International Agency for Research on Cancer, 150 Cours Albert Thomas, 69372 Lyon Cedex 08, France © International Agency for Research on Cancer, 2008-11-24 Distributed by WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]) Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturer’s products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The authors alone are responsible for the views expressed in this publication. The International Agency for Research on Cancer welcomes requests for permission to reproduce or translate its publications, in part or in full. -

Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors
Pharmaceutics 2011 , 3, 615-635; doi:10.3390/pharmaceutics3030615 OPEN ACCESS pharmaceutics ISSN 1999-4923 www.mdpi.com/journal/pharmaceutics Review Transporter-Mediated Drug Interaction Strategy for 5-Aminolevulinic Acid (ALA)-Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors Toshihisa Ishikawa 1,*, Kenkichi Takahashi 2, Naokado Ikeda 2, Yoshinaga Kajimoto 2, Yuichiro Hagiya 3, Shun-ichiro Ogura 3, Shin-ichi Miyatake 2 and Toshihiko Kuroiwa 2 1 Omics Science Center, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, 230-0045, Japan 2 Department of Neurosurgery, Osaka Medical College, Osaka 569-8686, Japan 3 Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama 226-8501, Japan * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel.: +81-45-503-9222; Fax: +81-45-503-9216. Received: 19 July 2011; in revised form: 16 August 2011 / Accepted: 9 September 2011 / Published: 14 September 2011 Abstract: Photodynamic diagnosis (PDD) is a practical tool currently used in surgical operation of aggressive brain tumors, such as glioblastoma. PDD is achieved by a photon-induced physicochemical reaction which is induced by excitation of protoporphyrin IX (PpIX) exposed to light. Fluorescence-guided gross-total resection has recently been developed in PDD, where 5-aminolevulinic acid (ALA) or its ester is administered as the precursor of PpIX. ALA induces the accumulation of PpIX, a natural photo-sensitizer, in cancer cells. Recent studies provide evidence that adenosine triphosphate (ATP)-binding cassette (ABC) transporter ABCG2 plays a pivotal role in regulating the cellular accumulation of porphyrins in cancer cells and thereby affects the efficacy of PDD. -

Bilirubin Secretion, Jaundice and Evaluation of Liver Function
Bilirubin Secretion, Jaundice and Evaluation of Liver Function Howard J. Worman, M. D. Evaluation of Liver Disease and Hepatic Function History Physical Examination Laboratory Tests Sometimes Radiological/Nuclear Medicine Sometimes Liver Biopsy 1 Jaundice occurs as a result of excess bilirubin in the blood. It is a hallmark of liver disease but not always present in liver disease. Jaundice occurs when the liver fails to adequately secrete bilirubin from the blood into the bile. To understand how jaundice occurs, you must first understand bilirubin synthesis, metabolism and secretion. 2 Heme Oxygenase Schuller et al. Nature Structural Biology 6, 860 - 867 (1999) Bilivirdin Reductase Kikuchi et al. Nature Structural Biology 8, 221 - 225 (2001) 3 Bilirubin is frequently depicted as a linear tetrapyrrole. However, intramolecular hydrogen bonding fixes it in a rigid structure that blocks exposure of its polar groups to aqueous solvents, making it very insoluble in blood. 4 Bilirubin in Blood is Bound to Albumin: Uptake into Hepatocyte at Basolateral (Sinusoidal) Membrane Some OATP-C? bilirubin stored in cytosol bound to proteins Bilurubin UDP-glucuronosyltransferase is localized to the endo- plasmic reticulum; it catalyzes conjugation to a diglucuronide, making it more water soluble. A: Labeling of periphery of cell hepatocyte nucleus B: Labeling of ER with antibody to UDP-glucuronosyltransferase 5 Alternative RNA splicing of different first exons of UGT1 gives different isoforms with different substrate specificities, some for bilirubin and others -

UVB-Induced Conversion of 7-Dehydrocholesterol to 1Α,25-Dihydroxyvitamin D3 in an in Vitro Human Skin Equivalent Model
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector UVB-Induced Conversion of 7-Dehydrocholesterol to 1a,25- Dihydroxyvitamin D3 in an In Vitro Human Skin Equivalent Model Bodo Lehmann, Thurid Genehr, Peter Knuschke, Jens Pietzsch,* and Michael Meurer Department of Dermatology, *Institute and Policlinic of Clinical Metabolic Research, Carl Gustav Carus Medical School, Dresden University of Technology, Germany We have previously shown that keratinocytes in vitro hydroxyvitamin D3, and ultimately calcitriol in the can convert biologically inactive vitamin D3 to the femtomolar range. Unirradiated cultures and irradi- hormone calcitriol (1a,25-dihydroxyvitamin D3). ated cultures without keratinocytes generated no cal- This study was initiated to test whether the ultra- citriol. Irradiation of skin equivalents at wavelengths violet-B-induced photolysis of provitamin D3 (7-de- > 315 nm generated no or only trace amounts of cal- hydrocholesterol), which results in the formation of citriol. The ultraviolet-B-triggered conversion of vitamin D3, can generate calcitriol in an in vivo-like 7-dehydrocholesterol to calcitriol was strongly human skin equivalent model made of ®broblasts in inhibited by ketoconazole indicating the involvement a collagen matrix as the dermal component and of P450 mixed function oxidases. The amount of cal- keratinocytes as the epidermal component. Cultures citriol generated was dependent on the 7-dehydro- were preincubated with increasing concentrations cholesterol concentration, on wavelength, and on of 7-dehydrocholesterol (0.53±5.94 nmol per cm2 ultraviolet B dose. Hence, keratinocytes in the pres- human skin equivalent) at 37°C and irradiated with ence of physiologic concentrations of 7-dehydro- monochromatic ultraviolet B at wavelengths ranging cholesterol and irradiated with therapeutic doses of from 285 to 315 nm (effective ultraviolet doses 7.5± ultraviolet B may be a potential source of biologic- 45 mJ per cm2). -

Adjuvant Therapy with High Dose Vitamin D Following Primary Treatment of Melanoma at High Risk of Recurrence
Saw et al. BMC Cancer 2014, 14:780 http://www.biomedcentral.com/1471-2407/14/780 STUDY PROTOCOL Open Access Adjuvant therapy with high dose vitamin D following primary treatment of melanoma at high risk of recurrence: a placebo controlled randomised phase II trial (ANZMTG 02.09 Mel-D) Robyn PM Saw1,2,3,6*, Bruce K Armstrong4, Rebecca S Mason5, Rachael L Morton4,6, Kerwin F Shannon1,3,6, Andrew J Spillane1,6,7, Jonathan R Stretch1,2,3,6 and John F Thompson1,2,3,6 Abstract Background: Patients with primary cutaneous melanomas that are ulcerated and >2 mm in thickness, >4 mm in thickness and those with nodal micrometastases at diagnosis, have few options for adjuvant treatment. Recent studies have suggested a role for vitamin D to delay melanoma recurrence and improve overall prognosis. Methods/Design: This is a pilot placebo-controlled randomised phase II trial to assess the feasibility, safety and toxicity of an oral loading dose of Vitamin D (500,000 IU) followed by an oral dose of 50,000 IU of Vitamin D monthly for 2 years in patients who have been treated for cutaneous melanoma by wide excision of the primary. Patients aged 18 – 79 years who have completed primary surgical treatment and have Stage IIb, IIc, IIIa (N1a, N2a) or IIIb (N1a, N2a) disease are eligible for randomisation 2:1 to active treatment or placebo. The primary endpoints are sufficiency of dose, adherence to study medication and safety of the drug. The secondary endpoints are participation and progression free survival. The study has been approved by the Ethics Review Committee (RPAH Zone) of the Sydney Local Health District, protocol number X09-0138. -

Metabolism of Vitamins
Metabolism of vitamins Arash Azarfar Lorestan University McDowell, L. R. 2000. Vitamins in Animal and Human Nutrition, 2nd ed., Iowa State University Press, Ames, IA Bender, 2003, Nutritional biochemistry of vitamins, 2nd edition, Cambridge, UK. Berdanier,C.D. 2000, Advanced nutrition micronutrients, CRC Press. Lorestan University ویتامین ها Vitamins Vitamins are substances which are involved in: • Gene expression (A, D, B1, B12) • Structural role in visual pigments (A as retinol) • As antioxidant (A, E, C) • As enzyme cofactors (B group, K) The first identified vitamin was identified by Funk(1921). ‘Vital amine’ or ;vitamine’ ‘Vitamin’ Lorestan University ویتامین ها : Vitamin : fat soluble type Names Functions A, retinoic acid, retinol, Vision, retinaldehyde Maintenance of epithelial cells Reproduction, Growth, D, Phosphocalcium metabolism, cholecalciferol,ergocalciferol Growth, Reproduction, E, α-, β-, γ-, tocopherols Biological antioxidant, phospholipids' membrane stability, Immunomodulation K, menadion, phylloquinone, Cofactor in coagulation menaquinone Lorestan University ویتامین ها Vitamins: water soluble Names Functions Thiamin, B1, aneurin Coenzyme in oxidative Decarboxylation, Role in neurophysiology Ribofelavin, B2 Intermediary in the transfer of electrons in biological oxidation- reduction reactions Niacin , B3, nicotinamide, pp Constituent of coenzyme NAD factor and NADP in carbohydrate, protein and fat metabolism Panthotenic acid, B5 Coenzyme A precursor B6, pyridoxine, pyridoxal, Amino acid metabolism, pyridoxamine Formation -

Inherited Disorders of Bilirubin Clearance N
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Hofstra Northwell Academic Works (Hofstra Northwell School of Medicine) Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2015 Inherited disorders of bilirubin clearance N. Memon B. I. Weinberger Zucker School of Medicine at Hofstra/Northwell T. Hegyi L. M. Aleksunes Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Pediatrics Commons Recommended Citation Memon N, Weinberger B, Hegyi T, Aleksunes L. Inherited disorders of bilirubin clearance. 2015 Jan 01; 79(3):Article 2776 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/2776. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. For more information, please contact [email protected]. HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Pediatr Manuscript Author Res. Author manuscript; Manuscript Author available in PMC 2016 April 05. Published in final edited form as: Pediatr Res. 2016 March ; 79(3): 378–386. doi:10.1038/pr.2015.247. Inherited Disorders of Bilirubin Clearance Naureen Memon1,*, Barry I Weinberger2, Thomas Hegyi1, and Lauren M Aleksunes3 1Department of Pediatrics, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA 2Department of Pediatrics, Cohen Children’s Medical Center of New York, New Hyde Park, NY, USA 3Department of Pharmacology and Toxicology, Rutgers University, Piscataway, NJ, USA Abstract Inherited disorders of hyperbilirubinemia may be caused by increased bilirubin production or decreased bilirubin clearance. -

Novel Pharmacogenomic Markers of Irinotecan-Induced Severe Toxicity in Metastatic Colorectal Cancer Patients
Novel pharmacogenomic markers of irinotecan-induced severe toxicity in metastatic colorectal cancer patients Thèse Siwen Sylvialin Chen Doctorat en sciences pharmaceutiques Philosophiae doctor (Ph.D.) Québec, Canada © Siwen Sylvialin Chen, 2015 ii Résumé L’irinotécan est un agent de chimiothérapie largement utilisé pour le traitement de tumeurs solides, particulièrement pour le cancer colorectal métastatique (mCRC). Fréquemment, le traitement par l’irinotécan conduit à la neutropénie et la diarrhée, des effets secondaires sévères qui peuvent limiter la poursuite du traitement et la qualité de vie des patients. Plusieurs études pharmacogénomiques ont évalué les risques associés à la chimiothérapie à base d’irinotécan, en particulier en lien avec le gène UGT1A, alors que peu d’études ont examiné l’impact des gènes codant pour des transporteurs. Par exemple, le marqueur UGT1A1*28 a été associé à une augmentation de 2 fois du risque de neutropénie, mais ce marqueur ne permet pas de prédire la toxicité gastrointestinale ou l’issue clinique. L’objectif de cette étude était de découvrir de nouveaux marqueurs génétiques associés au risque de toxicité induite par l’irinotécan, en utilisant une stratégie d’haplotype/SNP-étiquette permettant de maximiser la couverture des loci génétiques ciblés. Nous avons analysé les associations génétiques des loci UGT1 et sept gènes codants pour des transporteurs ABC impliqués dans la pharmacocinétique de l’irinotécan, soient ABCB1, ABCC1, ABCC2, ABCC5, ABCG1, ABCG2 ainsi que SLCO1B1. Les profils de 167 patients canadiens atteints de mCRC sous traitement FOLFIRI (à base d’irinotécan) ont été examinés et les marqueurs significatifs ont par la suite été validés dans une cohorte indépendante de 250 patients italiens.