Inherited Disorders of Bilirubin Clearance N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neonatal Hyperbilirubinemia
Clinical Chemistry Trainee Council Pearls of Laboratory Medicine www.traineecouncil.org TITLE: Neonatal Hyperbilirubinemia PRESENTER: Linnea M. Baudhuin, Ph.D., DABMG Slide 1: Title slide Slide 2: Bilirubin is an orange‐yellow pigment derived from the degradation of the heme moiety of hemoproteins, particularly the hemoglobin of mature circulating erythrocytes. Hemo oxygenase is the enzyme initially responsible for the degradation of hemoglobin, producing biliverdin and carbon monoxide. Biliverdin reductase reduces the green‐pigmented biliverdin to bilirubin. Bilirubin production primarily occurs in the liver, but also can occur in the spleen and bone marrow, and as it is produced in the unconjugated form, bilirubin is highly insoluble. Bilirubin is conjugated in the liver, which allows excretion in the bile and urine. Bilirubin is mainly conjugated to glucuronic acid by uridine diphosphate glucuronosyl transferase 1A1, or UGT1A1. Slide 3: Bilirubin is photosensitive because light causes configurational and structural changes to unconjugated bilirubin. The structure of bilirubin, as established by x‐ray crystallography, is a ridge‐tiled configuration stabilized by six intramolecular hydrogen bonds. These six intramolecular hydrogen bonds stabilize the Z‐Z structure of unconjugated bilirubin, rendering it insoluble. Rotation and/or breakage of the bonds on carbon atoms 5 and 15 lead to more open E‐Z‐ and Z‐E‐bilirubin structures, which are more water soluble. The sensitivity of bilirubin to light is the basis for phototherapy in individuals with unconjugated hyperbilirubinemia. Clinical measurement of bilirubin can also be affected due to this light sensitivity. Slide 4: Four fractions of bilirubin can be revealed upon open‐column chromatographic separation that does not involve deproteinization. -
Baseline Levels of Endogenous Compounds in the Caucasian Population S
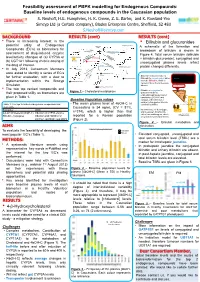
Feasibility assessment of PBPK modelling for Endogenous Compounds: Baseline levels of endogenous compounds in the Caucasian population S. Neuhoff, H.E. Humphries, H. K. Crewe, Z. E. Barter, and K. Rowland-Yeo Simcyp Ltd (a Certara company), Blades Enterprise Centre, Sheffield, S2 4SU [email protected] BACKGROUND RESULTS (cont) RESULTS (cont) • There is increasing interest in the • Bilirubin and glucuronides 27-Hydroxycholesterol potential utility of Endogenous Cholesterol 1g/day A schematic of the formation and Compounds (EC’s) as biomarkers for CYP7A1 (liver) 500 mg/day CYP27A1 (many tissues) breakdown of bilirubin is shown in assessment of drug-induced enzyme 19 mg/day Figure 4. Total serum bilirubin (bilirubin 7α-Hydroxycholesterol level/activity changes of (a) CYP3A or CYP3A4 + bilirubin glucuronide), conjugated and Bile Acids (b) UGT1A1 following chronic dosing of 4-Hydroxycholesterol unconjugated plasma levels reflect the drug of interest. CYP27A1 (liver and extrahepatic) 24S-Hydroxycholesterol protein changes differently. • In July 2013, Consortium Members Pregnenolone were asked to identify a series of EC’s 4,27-Dihydroxycholesterol Macrophage CYP27A1 • Biliverdin reductase reduces Heme Hemeoxygenase for further evaluation, with a view to 4,24-Dihydroxycholesterol Biliverdin to unconjugated, water- Biliverdin 4,7α-Dihydroxycholesterol insoluble Bilirubin, which is Biliverdin reductase implementation within the Simcyp Bilirubin carried in blood bound to serum Unconjugated bilirubin Simulator. albumin complexed with albumin 3 -

Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors
Pharmaceutics 2011 , 3, 615-635; doi:10.3390/pharmaceutics3030615 OPEN ACCESS pharmaceutics ISSN 1999-4923 www.mdpi.com/journal/pharmaceutics Review Transporter-Mediated Drug Interaction Strategy for 5-Aminolevulinic Acid (ALA)-Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors Toshihisa Ishikawa 1,*, Kenkichi Takahashi 2, Naokado Ikeda 2, Yoshinaga Kajimoto 2, Yuichiro Hagiya 3, Shun-ichiro Ogura 3, Shin-ichi Miyatake 2 and Toshihiko Kuroiwa 2 1 Omics Science Center, RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, 230-0045, Japan 2 Department of Neurosurgery, Osaka Medical College, Osaka 569-8686, Japan 3 Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama 226-8501, Japan * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel.: +81-45-503-9222; Fax: +81-45-503-9216. Received: 19 July 2011; in revised form: 16 August 2011 / Accepted: 9 September 2011 / Published: 14 September 2011 Abstract: Photodynamic diagnosis (PDD) is a practical tool currently used in surgical operation of aggressive brain tumors, such as glioblastoma. PDD is achieved by a photon-induced physicochemical reaction which is induced by excitation of protoporphyrin IX (PpIX) exposed to light. Fluorescence-guided gross-total resection has recently been developed in PDD, where 5-aminolevulinic acid (ALA) or its ester is administered as the precursor of PpIX. ALA induces the accumulation of PpIX, a natural photo-sensitizer, in cancer cells. Recent studies provide evidence that adenosine triphosphate (ATP)-binding cassette (ABC) transporter ABCG2 plays a pivotal role in regulating the cellular accumulation of porphyrins in cancer cells and thereby affects the efficacy of PDD. -

Bilirubin Secretion, Jaundice and Evaluation of Liver Function
Bilirubin Secretion, Jaundice and Evaluation of Liver Function Howard J. Worman, M. D. Evaluation of Liver Disease and Hepatic Function History Physical Examination Laboratory Tests Sometimes Radiological/Nuclear Medicine Sometimes Liver Biopsy 1 Jaundice occurs as a result of excess bilirubin in the blood. It is a hallmark of liver disease but not always present in liver disease. Jaundice occurs when the liver fails to adequately secrete bilirubin from the blood into the bile. To understand how jaundice occurs, you must first understand bilirubin synthesis, metabolism and secretion. 2 Heme Oxygenase Schuller et al. Nature Structural Biology 6, 860 - 867 (1999) Bilivirdin Reductase Kikuchi et al. Nature Structural Biology 8, 221 - 225 (2001) 3 Bilirubin is frequently depicted as a linear tetrapyrrole. However, intramolecular hydrogen bonding fixes it in a rigid structure that blocks exposure of its polar groups to aqueous solvents, making it very insoluble in blood. 4 Bilirubin in Blood is Bound to Albumin: Uptake into Hepatocyte at Basolateral (Sinusoidal) Membrane Some OATP-C? bilirubin stored in cytosol bound to proteins Bilurubin UDP-glucuronosyltransferase is localized to the endo- plasmic reticulum; it catalyzes conjugation to a diglucuronide, making it more water soluble. A: Labeling of periphery of cell hepatocyte nucleus B: Labeling of ER with antibody to UDP-glucuronosyltransferase 5 Alternative RNA splicing of different first exons of UGT1 gives different isoforms with different substrate specificities, some for bilirubin and others -

Dubin-Johnson Syndrome with Some Unusual Features in a Chinese Family
Arch Dis Child: first published as 10.1136/adc.54.7.529 on 1 July 1979. Downloaded from Archives of Disease in Childhood, 1979, 54, 529-533 Dubin-Johnson syndrome with some unusual features in a Chinese family N. S. LO, C. W. CHAN, AND J. H. HUTCHISON University Departments ofPaediatrics and Pathology, Queen Mary Hospital, Hong Kong SUMMARY Three cases of chronic nonhaemolytic jaundice with conjugated bilirubin in the serum an described in a Chinese family. Bromsulphthalein excretion tests gave results typical of the Dubin- Johnson syndrome. Liver histology in the proband showed cytoplasmic pigment of the lipofuscin- melanin variety, and intravenous cholecystography failed to show visualisation of the gallbladder. Unusual findings included onset during the neonatal period in the proband and the presence of some iron pigment in the hepatic cells with a little canalicular cholestasis. It is suggested that the infant may have had a concomitant nonspecific hepatitis. These cases are regarded as belonging to a disease group in which the Dubin-Johnson syndrome is at one end of a spectrum. The mode of inheritance is discussed. The hereditary defects in bilirubin metabolism are United Christian Hospital, Hong Kong on 22 rare and incompletely understood causes ofjaundice. March 1978. The birthweight was 3 58 kg. There They can be divided into two groups according to was no history of maternal illness, drug ingestion, or whether the raised serum bilirubin level occurs only in x-irradiation. Jaundice was noted on day 2 when the unconjugated form as in Gilbert's disease (Berk the total serum bilirubin (SB) level was 225 * 7 ,tmol/l et al., 1970) and Crigler-Najjar disease (Crigler and (13 2 mg/100 ml). -

ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries
18 PRACTICE GUIDELINES CME ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries P a u l Y. K w o , M D , F A C G , F A A S L D 1 , Stanley M. Cohen , MD, FACG, FAASLD2 and Joseph K. Lim , MD, FACG, FAASLD3 Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defi ned as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defi ned as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. The evaluation of hepatocellular injury includes testing for viral hepatitis A, B, and C, assessment for nonalcoholic fatty liver disease and alcoholic liver disease, screening for hereditary hemochromatosis, autoimmune hepatitis, Wilson’s disease, and alpha-1 antitrypsin defi ciency. In addition, a history of prescribed and over-the-counter medicines should be sought. For the evaluation of an alkaline phosphatase elevation determined to be of hepatic origin, testing for primary biliary cholangitis and primary sclerosing cholangitis should be undertaken. -

Novel Pharmacogenomic Markers of Irinotecan-Induced Severe Toxicity in Metastatic Colorectal Cancer Patients
Novel pharmacogenomic markers of irinotecan-induced severe toxicity in metastatic colorectal cancer patients Thèse Siwen Sylvialin Chen Doctorat en sciences pharmaceutiques Philosophiae doctor (Ph.D.) Québec, Canada © Siwen Sylvialin Chen, 2015 ii Résumé L’irinotécan est un agent de chimiothérapie largement utilisé pour le traitement de tumeurs solides, particulièrement pour le cancer colorectal métastatique (mCRC). Fréquemment, le traitement par l’irinotécan conduit à la neutropénie et la diarrhée, des effets secondaires sévères qui peuvent limiter la poursuite du traitement et la qualité de vie des patients. Plusieurs études pharmacogénomiques ont évalué les risques associés à la chimiothérapie à base d’irinotécan, en particulier en lien avec le gène UGT1A, alors que peu d’études ont examiné l’impact des gènes codant pour des transporteurs. Par exemple, le marqueur UGT1A1*28 a été associé à une augmentation de 2 fois du risque de neutropénie, mais ce marqueur ne permet pas de prédire la toxicité gastrointestinale ou l’issue clinique. L’objectif de cette étude était de découvrir de nouveaux marqueurs génétiques associés au risque de toxicité induite par l’irinotécan, en utilisant une stratégie d’haplotype/SNP-étiquette permettant de maximiser la couverture des loci génétiques ciblés. Nous avons analysé les associations génétiques des loci UGT1 et sept gènes codants pour des transporteurs ABC impliqués dans la pharmacocinétique de l’irinotécan, soient ABCB1, ABCC1, ABCC2, ABCC5, ABCG1, ABCG2 ainsi que SLCO1B1. Les profils de 167 patients canadiens atteints de mCRC sous traitement FOLFIRI (à base d’irinotécan) ont été examinés et les marqueurs significatifs ont par la suite été validés dans une cohorte indépendante de 250 patients italiens. -

Mechanism of Bilirubin Diglucuronide Formation in Intact Rats: BILIRUBIN
Mechanism of Bilirubin Diglucuronide Formation in Intact Rats BILIRUBIN DIGLUCURONIDE FORMATION IN VIVO NORBERT BLANCKAERT, JOHN GOLLAN, and RUDI SCHMID, Department of Medicine and Liver Center, University of California School of Medicine, San Francisco, California 94143; Laboratory of Hepatology, Department of Medical Research, University of Leuven, B-3000, Leuven, Belgium A B S T R A C T Although it is well established that bili- ['4C]monoglucuronide, no transfer or exchange of the rubin monoglucuronide is formed in the liver from bili- ['4C]glucuronosyl group between injected and en- rubin by a microsomal bilirubin uridine diphosphate dogenously produced bilirubin monoglucuronide (UDP)-glucuronosyltransferase, the subcellular site of could be detected in the excreted bilirubin diglucuron- conversion of monoglucuronide to diglucuronide and ide. Third, in homozygous Gunn rats, injected "4C- the molecular mechanism involved in diglucuronide labeled or unlabeled bilirubin mono- or digluc- synthesis have not been identified. Based on in vitro uronides were excreted in bile unchanged (except that studies, it has been proposed that two fundamentally diglucuronide was hydrolyzed to a minor degree). This different enzyme systems may be involved in di- indicates that Gunn rats, which lack bilirubin UDP- glucuronide synthesis in rat liver: (a) a microsomal glucuronosyltransferase activity, are unable to convert UDP-glucuronosyltransferase system requiring UDP- injected monoglucuronide to diglucuronide. Collec- glucuronic acid as sugar donor or (b) a transglucuroni- tively, these findings establish that a transglucuronida- dation mechanism that involves transfer of a glucurono- tion mechanism is not operational in vivo and support syl residue from one monoglucuronide molecule to the concept that bilirubin diglucuronide is formed by another, catalyzed by a liver plasma membrane en- a microsomal UDP-glucuronosyltransferase system. -

Wilson Disease Α1-Anti Antitrypsin Deficiency Hereditary Hyperbilirubinemia
Wilson Disease α1-Anti Antitrypsin Deficiency Hereditary Hyperbilirubinemia NEIL CRITTENDEN OCTOBER 29, 2015 Wilson Disease Wilson Disease: Autosomal Recessive Disorder of copper overload Incidence 1 in 30,000 Copper accumulation in the liver, brain, kidney and cornea Related to the gene ATP7B -> mutated ATP7B protein (an ATPase that can’t excrete Cu) Symptom onset range 3 to 55 years May present with isolated elevation of LFT’s, progressive neurologic/psychiatric disorder or isolated acute hemolysis With fulminant hepatic failure, acute intravascular hemolysis is usually present Wilson Disease: Diagnosis J. Korman Hepatology 2008; 48:1167-1174 J. Korman Hepatology 2008; 48:1167-1174 J. Korman Hepatology 2008; 48:1167-1174 Wilson Disease: Chronic Diagnosis, Initial Clues Hepatic Inflammation, AST:ALT usually >2 CBC may show anemia, (Coombs-Negative hemolytic anemia) Low serum copper concentration Low serum uric acid and phosphate (renal tubular dysfunction) Wilson Disease: Chronic Diagnosis Ocular Slit-Lamp Examination Ceruloplasmin < 20 mg/dL DDx: Wilson (homozygous vs. heterozygous, other chronic liver disease, intestinal malabsorption, nephrosis, malnutrition, hereditary aceruloplasminemia) 24-hour urinary copper excretion (preferably 3 separate), usually 2-3 x Normal, or 100 ug/day Hepatic tissue copper concentration > 250 ug/g dry weight of liver is diagnostic of Wilson disease Aside on Liver Biopsy Require Cu Free Needle (BioPince used at UofL and Jewish Hospital) Collect in a plastic container which has been rinsed -

International Symposium on Bile Pigments
Gut: first published as 10.1136/gut.7.6.706 on 1 December 1966. Downloaded from Gut, 1966, 7, 706 International symposium on bile pigments An international symposium on bile pigments was 100 ml. In normal human adults (blood bank doners) the held at the Royal Free Hospital, London, on 11 and serum bilirubin glucuronide concentrations ranged from 12 July 1966. 5 to 25 ,g. per 100 ml. Male donors had significantly higher serum levels than females. ORIGIN OF BILE PIGMENTS B. NOIR (Buenos Aires) presented chromatographic evi- L G. ISRAELS (Winnipeg) produced evidence that the dence which showed that 10 to 15 % of the conjugated bile early labelled fraction of bilirubin which could be demon- pigments was present as bilirubin sulphate. strated using isotopically labelled glycine was composed of two components, one related to erythropoiesis and the E. TALAFANT (Czechoslovakia) studied the nature of other apparently unrelated to erythropoiesis and probably ether-extractable bilirubin in obstructive jaundice caused of hepatic origin. by carcinoma. He postulated that this was a complex of bilirubin glucuronide with an unknown lipid substance. S. SCHWARTZ (Minneapolis) emphasized that the early labelled bilirubin fraction probably had many compon- J. D. OSTROW (Cleveland) examined the yellow pigments ents each with its own turnover rate and that this made in bile of the Gunn rat and showed that they could not be quantitation ofeach component all the more difficult. identified with the yellow pigments formed from the photooxidative breakdown of bilirubin. S. H. ROBINSON (Boston) described a new experimental technique for studying the early labelled component of M. -

Molecular Genetics of Variegate Porphyria in Finland
Department of Medicine, Division of Endocrinology University of Helsinki, Finland MOLECULAR GENETICS OF VARIEGATE PORPHYRIA IN FINLAND Mikael von und zu Fraunberg Academic dissertation To be presented with the permission of the Medical Faculty of the University of Helsinki, for public examination in Auditorium II, Haartmaninkatu 8, Biomedicum Helsinki, on March 22th 2003, at 12:00 noon Helsinki 2003 SUPERVISED BY Docent Raili Kauppinen, M.D., Ph.D. Department of Medicine Division of Endocrinology University of Helsinki Finland REVIEWED BY Professor Richard J. Hift, M.D., Ph.D. Department of Medicine Lennox Eales Porphyria Laboratory MRC/UCT Liver Research Centre University of Cape Town South Africa and Professor Eeva-Riitta Savolainen, M.D., Ph.D. Department of Clinical Chemistry University of Oulu Finland OFFICIAL OPPONENT: Docent Katriina Aalto-Setälä, M.D., Ph.D. Department of Medicine University of Tampere Finland © Mikael von und zu Fraunberg ISBN 952-91-5643-X (paperback) ISBN 952-10-0970-5 (pdf) http://ethesis.helsinki.fi Helsinki 2003 Yliopistopaino to my family CONTENTS SUMMARY............................................................................................................... 6 ORIGINAL PUBLICATIONS................................................................................... 8 ABBREVIATIONS.................................................................................................... 9 INTRODUCTION ................................................................................................... 10 REVIEW -

7 Bilirubin Metabolism
#7 Bilirubin metabolism Objectives : ● Definition of bilirubin ● The normal plasma concentration of total bilirubin ● Bilirubin metabolism : - Bilirubin formation - Transport of bilirubin in plasma - Hepatic bilirubin transport - Excretion through intestine ● Other substances conjugated by glucuronyl transferase. ● Differentiation between conjugated & unconjugated bilirubin ● Other substances excreted in the bile ● Definition of Jaundice ● Classification of jaundice ( Prehepatic / Hepatic / poat-hepatic ). Doctors’ notes Extra Important Resources: 435 Boys’ & Girls’ slides | Guyton and Hall 12th & 13th edition Editing file [email protected] 1 Overview- mind map Porphyrin Metabolism (Boys’ slides) : ● Porphyrins are cyclic compounds that readily bind metal ions usually Fe2+ or +3 Fe which can carry O2. ● Porphyrins are heterocyclic macrocycles composed of four modified pyrrole (a colorless, toxic, liquid, five-membered ring compound, C4 H5 N) subunits interconnected at their α carbon atoms via methine bridges (=CH-). ● The most prevalent porphyrin in the human is heme, which consists of one ferrous (Fe2+ ) iron ion coordinated in the center of tetrapyrrole ring of protoporphyrin IX. ● Structure of Hemoglobin showing the polypeptides backbone that are composed of four subunits: 2 α and 2 β subunits. Every subunit is consisted of one ferrous (Fe2+ ) iron ion coordinated in the center porphyrin compound. The most prevalent porphyrin in the human is heme Definition of bilirubin : ● Bilirubin is the end product of heme degradation derived from breakdown senescent (aging) erythrocytes by mononuclear phagocytes system specially in the spleen, liver and bone marrow. (It is the water insoluble breakdown product of normal heme catabolism). ● Bilirubin is the greenish yellow pigment excreted in bile, urine and feces. ● The major pigment present in bile is the orange compound bilirubin.