Functions of the Intermediate Filament Cytoskeleton in the Eye Lens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Isyte: Integrated Systems Tool for Eye Gene Discovery
Lens iSyTE: Integrated Systems Tool for Eye Gene Discovery Salil A. Lachke,1,2,3,4 Joshua W. K. Ho,1,4,5 Gregory V. Kryukov,1,4,6 Daniel J. O’Connell,1 Anton Aboukhalil,1,7 Martha L. Bulyk,1,8,9 Peter J. Park,1,5,10 and Richard L. Maas1 PURPOSE. To facilitate the identification of genes associated ther investigation. Extension of this approach to other ocular with cataract and other ocular defects, the authors developed tissue components will facilitate eye disease gene discovery. and validated a computational tool termed iSyTE (integrated (Invest Ophthalmol Vis Sci. 2012;53:1617–1627) DOI: Systems Tool for Eye gene discovery; http://bioinformatics. 10.1167/iovs.11-8839 udel.edu/Research/iSyTE). iSyTE uses a mouse embryonic lens gene expression data set as a bioinformatics filter to select candidate genes from human or mouse genomic regions impli- ven with the advent of high-throughput sequencing, the cated in disease and to prioritize them for further mutational Ediscovery of genes associated with congenital birth defects and functional analyses. such as eye defects remains a challenge. We sought to develop METHODS. Microarray gene expression profiles were obtained a straightforward experimental approach that could facilitate for microdissected embryonic mouse lens at three key devel- the identification of candidate genes for developmental disor- opmental time points in the transition from the embryonic day ders, and, as proof-of-principle, we chose defects involving the (E)10.5 stage of lens placode invagination to E12.5 lens primary ocular lens. Opacification of the lens results in cataract, a leading cause of blindness that affects 77 million persons and fiber cell differentiation. -

A New Mutation in BFSP2 (G1091A) Causes Autosomal Dominant Congenital Lamellar Cataracts
Molecular Vision 2008; 14:1906-1911 <http://www.molvis.org/molvis/v14/a226> © 2008 Molecular Vision Received 17 August 2008 | Accepted 18 October 2008 | Published 24 October 2008 A new mutation in BFSP2 (G1091A) causes autosomal dominant congenital lamellar cataracts Xu Ma,1,2,3 Fei-Feng Li,1,2 Shu-Zhen Wang,4 Chang Gao,1,2 Meng Zhang,2 Si-Quan Zhu4 (The first three authors contributed equally to this work.) 1Graduate School, Peking Union Medical College, Beijing, China; 2Department of Genetics, National Research Institute for Family Planning, Beijing, China; 3WHO Collaborative Center for Research in Human Reproduction, Beijing, China; 4Beijing Tongren Eye Center, Capital Medical University, Beijing, China Purpose: We sought to identify the genetic defect in a four-generation Chinese family with autosomal dominant congenital lamellar cataracts and demonstrate the functional analysis with biosoftware of a candidate gene in the family. Methods: Family history data were recorded. Clinical and ophthalmologic examinations were performed on family members. All the members were genotyped with microsatellite markers at loci considered to be associated with cataracts. Two-point LOD scores were calculated by using the Linkage Software after genotyping. A mutation was detected by using gene-specific primers in direct sequencing. Wild type and mutant proteins were analyzed with Online Bio-Software. Results: Affected members of this family had lamellar cataracts. Linkage analysis was obtained at markers D3S2322 (LOD score [Z]=7.22, recombination fraction [θ]=0.0) and D3S1541 (Z=5.42, θ=0.0). Haplotype analysis indicated that the cataract gene was closely linked to these two markers. -

Identification of the Binding Partners for Hspb2 and Cryab Reveals
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2013-12-12 Identification of the Binding arP tners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non- Redundant Roles for Small Heat Shock Proteins Kelsey Murphey Langston Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Microbiology Commons BYU ScholarsArchive Citation Langston, Kelsey Murphey, "Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins" (2013). Theses and Dissertations. 3822. https://scholarsarchive.byu.edu/etd/3822 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Julianne H. Grose, Chair William R. McCleary Brian Poole Department of Microbiology and Molecular Biology Brigham Young University December 2013 Copyright © 2013 Kelsey Langston All Rights Reserved ABSTRACT Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactors and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston Department of Microbiology and Molecular Biology, BYU Master of Science Small Heat Shock Proteins (sHSP) are molecular chaperones that play protective roles in cell survival and have been shown to possess chaperone activity. -
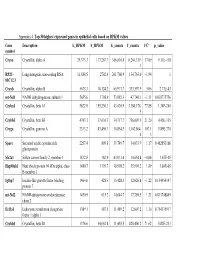
Appendix 4. Top 50 Highest Expressed Genes in Epithelial Cells Based on RPKM Values
Appendix 4. Top 50 highest expressed genes in epithelial cells based on RPKM values Gene Description E_RPKM F_RPKM E_counts F_counts FC* p_value symbol Cryaa Crystallin, alpha A 29,373.3 177,267.7 366,616.4 6,264,319. 17.09 9.11E-118 1 RP23– Long intergenic non-coding RNA 11,888.5 2702.4 261,760.9 134,763.0 −1.94 1 81C12.3 Cryab Crystallin, alpha B 5673.3 10,124.2 65,971.7 333,597.9 5.06 2.71E-43 mt-Nd1 NADH dehydrogenase, subunit 1 5655.6 1798.9 53,082.3 47,748.1 −1.11 0.838775756 Cryba1 Crystallin, beta A1 5622.0 155,230.3 43,420.9 3,380,176. 77.85 1.34E-240 5 Crybb3 Crystallin, beta B3 4743.1 37,636.3 34,717.7 736,007.9 21.20 4.45E-135 Cryga Crystallin, gamma A 2333.2 83,496.3 10,854.5 1,162,864. 107.1 5.89E-270 6 3 Sparc Secreted acidic cysteine rich 2257.4 809.8 39,749.7 34,033.9 −1.17 0.462853166 glycoprotein Slc2a1 Solute carrier family 2, member 1 1832.8 162.9 43,031.4 10,654.8 −4.04 1.67E-05 Hsp90ab1 Heat shock protein 90 kDa alpha, class 1480.7 1139.7 18,998.2 35,901.2 1.89 3.84E-05 B member 1 Igfbp7 Insulin-like growth factor binding 1464.6 428.3 15,428.3 12,626.8 −1.22 0.154954147 protein 7 mt-Nd2 NADH-ubiquinone oxidoreductase 1450.9 615.2 14,644.7 17,789.5 1.21 0.833748849 chain 2 Eef1a1 Eukaryotic translation elongation 1389.1 587.5 11,489.2 12,607.2 1.10 0.754135917 factor 1 alpha 1 Crybb1 Crystallin, beta B1 1376.6 34,662.8 11,455.5 820,406.2 71.62 5.82E-233 Htra3 HtrA serine peptidase 3 1338.6 162.0 23,197.6 6433.9 −3.61 3.93E-05 Gnb2l1 Guanine nucleotide-binding protein 1293.3 670.1 14,495.1 21,652.1 1.49 0.001685952 -

CRYGB Rabbit Pab
Leader in Biomolecular Solutions for Life Science CRYGB Rabbit pAb Catalog No.: A14569 Basic Information Background Catalog No. Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. A14569 The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their Observed MW nuclei during development, these crystallins are made and then retained throughout 21kDa life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a Calculated MW superfamily. Alpha and beta families are further divided into acidic and basic groups. 20kDa Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Gamma-crystallins are a homogeneous group of Category highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. Four Primary antibody gamma-crystallin genes (gamma-A through gamma-D) and three pseudogenes (gamma-E, gamma-F, gamma-G) are tandemly organized in a genomic segment as a Applications gene cluster. Whether due to aging or mutations in specific genes, gamma-crystallins WB have been involved in cataract formation. Cross-Reactivity Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 1419 P07316 Immunogen Recombinant fusion protein containing a sequence corresponding to amino acids 80-135 of human CRYGB (NP_005201.2). Synonyms CRYGB;CRYG2;CTRCT39 Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. -

Localization of the Lens Intermediate Filament Switch by Imaging Mass Spectrometry
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.21.053793; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Localization of the Lens Intermediate Filament Switch by Imaging Mass Spectrometry Zhen Wang, Daniel J. Ryan, and Kevin L Schey* Department of Biochemistry, Vanderbilt University, Nashville, TN 37232 * To whom correspondence should be addressed Current address: Mass Spectrometry Research Center Vanderbilt University 465 21st Ave. So., Suite 9160 MRB III Nashville, TN 37232-8575 E-mail: [email protected] Phone: 615.936.6861 Fax: 615.343.8372 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.21.053793; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Imaging mass spectrometry (IMS) enables targeted and untargeted visualization of the spatial localization of molecules in tissues with great specificity. The lens is a unique tissue that contains fiber cells corresponding to various stages of differentiation that are packed in a highly spatial order. The application of IMS to lens tissue localizes molecular features that are spatially related to the fiber cell organization. Such spatially resolved molecular information assists our understanding of lens structure and physiology; however, protein IMS studies are typically limited to abundant, soluble, low molecular weight proteins. In this study, a method was developed for imaging low solubility cytoskeletal proteins in the lens; a tissue that is filled with high concentrations of soluble crystallins. -

Variants in PAX6, PITX3 and HSF4 Causing Autosomal Dominant Congenital Cataracts ✉ ✉ Vanita Berry 1,2 , Alex Ionides2, Nikolas Pontikos 1,2, Anthony T
www.nature.com/eye ARTICLE OPEN Variants in PAX6, PITX3 and HSF4 causing autosomal dominant congenital cataracts ✉ ✉ Vanita Berry 1,2 , Alex Ionides2, Nikolas Pontikos 1,2, Anthony T. Moore2, Roy A. Quinlan3 and Michel Michaelides 1,2 © Crown 2021 BACKGROUND: Lens development is orchestrated by transcription factors. Disease-causing variants in transcription factors and their developmental target genes are associated with congenital cataracts and other eye anomalies. METHODS: Using whole exome sequencing, we identified disease-causing variants in two large British families and one isolated case with autosomal dominant congenital cataract. Bioinformatics analysis confirmed these disease-causing mutations as rare or novel variants, with a moderate to damaging pathogenicity score, with testing for segregation within the families using direct Sanger sequencing. RESULTS: Family A had a missense variant (c.184 G>A; p.V62M) in PAX6 and affected individuals presented with nuclear cataract. Family B had a frameshift variant (c.470–477dup; p.A160R*) in PITX3 that was also associated with nuclear cataract. A recurrent missense variant in HSF4 (c.341 T>C; p.L114P) was associated with congenital cataract in a single isolated case. CONCLUSIONS: We have therefore identified novel variants in PAX6 and PITX3 that cause autosomal dominant congenital cataract. Eye; https://doi.org/10.1038/s41433-021-01711-x INTRODUCTION consistent with early developmental effects as would be Cataract the opacification of the eye lens is the most common, but anticipated for PAX6 and PITX3 transcription factors. Recently, treatable cause of blindness in the world (https://www.who.int/ we have found two novel mutations in the transcription factors publications-detail/world-report-on-vision). -

The Uvomorulin-Anchorage Protein a Catenin Is a Vinculin
Proc. Nail. Acad. Sci. USA Vol. 88, pp. 9156-9160, October 1991 Cell Biology The uvomorulin-anchorage protein a catenin is a vinculin homologue KURT HERRENKNECHT*, MASAYUKI OZAWA*t, CHRISTOPH ECKERSKORN*, FRIEDRICH LOTTSPEICHt, MARTIN LENTER*, AND ROLF KEMLER*§ *Max-Planck-Institut ffir Immunbiologie, FG Molekulare Embryologie, D-7800 Freiburg, Federal Republic of Germany; and tMax-Planck-Institut ffr Biochemie, D-8033 Martinsried, Federal Republic of Germany Communicated by Franqois Jacob, July 18, 1991 (receivedfor review June 25, 1991) ABSTRACT The cytoplasmic region of the Ca2+- domain is well conserved in other cadherins, it is possible that dependent cell-adhesion molecule (CAM) uvomorulin associ- catenins may also complex with other members of this gene ates with distinct cytoplasmic proteins with molecular masses family (13, 14). Here we have produced antibodies against a of 102, 88, and 80 kDa termed a, (3, and ycatenin, respectively. catenin and show that a catenin is indeed associated with This complex formation links uvomorulin to the actin filament cadherins from human, mouse, and Xenopus. We have network, which seems to be of primary importance for its cloned and sequenced¶ the cDNA coding for a catenin and cell-adhesion properties. We show here that antibodies against have established the primary protein structure. Sequence a catenin also immunoprecipitate complexes that contain hu- comparison reveals homology to vinculin, a well-known man N-cadherin, mouse P-cadherin, chicken A-CAM (adhe- adherens-type and focal contact protein. rens junction-specific CAM; also called N-cadherin) or Xeno- pus U-cadherin, demonstrating that a catenin is complexed with other cadherins. -

A Comprehensive Analysis of the Expression of Crystallins in Mouse Retina Jinghua Xi Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2003 A comprehensive analysis of the expression of crystallins in mouse retina Jinghua Xi Washington University School of Medicine in St. Louis Rafal Farjo University of Michigan - Ann Arbor Shigeo Yoshida University of Michigan - Ann Arbor Timothy S. Kern Case Western Reserve University Anand Swaroop University of Michigan - Ann Arbor See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Xi, Jinghua; Farjo, Rafal; Yoshida, Shigeo; Kern, Timothy S.; Swaroop, Anand; and Andley, Usha P., ,"A comprehensive analysis of the expression of crystallins in mouse retina." Molecular Vision.9,. 410-419. (2003). https://digitalcommons.wustl.edu/open_access_pubs/1801 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Jinghua Xi, Rafal Farjo, Shigeo Yoshida, Timothy S. Kern, Anand Swaroop, and Usha P. Andley This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/1801 Molecular Vision 2003; 9:410-9 <http://www.molvis.org/molvis/v9/a53> © 2003 Molecular Vision Received 28 May 2003 | Accepted 19 August 2003 | Published 28 August 2003 A comprehensive analysis of the expression of crystallins in mouse retina Jinghua Xi,1 Rafal Farjo,3 Shigeo Yoshida,3 Timothy S. Kern,5 Anand Swaroop,3,4 Usha P. Andley1,2 Departments of 1Ophthalmology and Visual Sciences and 2Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. -

Related Macular Degeneration and Cutis Laxa
UvA-DARE (Digital Academic Repository) Genetic studies of age-related macular degeneration Baas, D.C. Publication date 2012 Document Version Final published version Link to publication Citation for published version (APA): Baas, D. C. (2012). Genetic studies of age-related macular degeneration. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:05 Oct 2021 G������ S������ �� A��-������� M������ D����������� D����������� M������ G������ S������ �� A��-������� | 2012 D�������� C. B��� G������ S������ �� A��-������� M������ D����������� D�������� C. B��� cover.indd 1 31-10-12 08:36 Genetic Studies of Age-related Macular Degeneration Dominique C. Baas Chapter 0.indd 1 23-10-12 19:24 The research described in this thesis was conducted at the Netherlands Institute for Neuroscience (NIN), an institute of the Royal Netherlands Academy of Arts and Sciences, Department of Clinical and Molecular Ophthalmogenetics, Amsterdam, The Netherlands. -

Role of Active Contraction and Tropomodulins in Regulating Actin Filament Length and Sarcomere Structure in Developing Zebrafish Skeletal Muscle
ORIGINAL RESEARCH published: 31 March 2016 doi: 10.3389/fphys.2016.00091 Role of Active Contraction and Tropomodulins in Regulating Actin Filament Length and Sarcomere Structure in Developing Zebrafish Skeletal Muscle Lise Mazelet 1, Matthew O. Parker 2, Mei Li 3, Anders Arner 3 and Rachel Ashworth 4* 1 School of Biological and Chemical Sciences, Queen Mary, University of London, London, UK, 2 School of Health Sciences and Social Work, University of Portsmouth, Portsmouth, UK, 3 Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 4 The Blizard Institute/Institute of Health Sciences Education, Barts and The London School of Medicine and Dentistry, London, UK Whilst it is recognized that contraction plays an important part in maintaining the structure and function of mature skeletal muscle, its role during development remains undefined. In this study the role of movement in skeletal muscle maturation was investigated in intact zebrafish embryos using a combination of genetic and pharmacological approaches. An immotile mutant line (cacnb1ts25) which lacks functional voltage-gated calcium channels (dihydropyridine receptors) in the muscle and pharmacological immobilization of embryos Edited by: with a reversible anesthetic (Tricaine), allowed the study of paralysis (in mutants and Catherine Coirault, anesthetized fish) and recovery of movement (reversal of anesthetic treatment). The Institut National de la Santé et de la Recherche Médicale, France effect of paralysis in early embryos (aged between 17 and 24 hours post-fertilization, hpf) Reviewed by: on skeletal muscle structure at both myofibrillar and myofilament level was determined Corrado Poggesi, using both immunostaining with confocal microscopy and small angle X-ray diffraction.