Molecular Detection of Yaba Monkey Tumour Virus from a Vervet Monkey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHO | World Health Organization
WHO/CDS/CSR/99.1 Report of the meeting of the Ad Hoc Committee on Orthopoxvirus Infections. Geneva, Switzerland, 14-15 January 1999 World Health Organization Department of Communicable Disease Surveillance and Response This document has been downloaded from the WHO/CSR Web site. The original cover pages and lists of participants are not included. See http://www.who.int/emc for more information. © World Health Organization This document is not a formal publication of the World Health Organization (WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale nor for use in conjunction with commercial purposes. The views expressed in documents by named authors are solely the responsibility of those authors. The mention of specific companies or specific manufacturers' products does no imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Contents Introduction 1 Recent monkeypox outbreaks in the Democratic Republic of Congo 1 Review of the report of the 1994 Ad Hoc Committee on Orthopoxvirus Infections 2 Work in WHO Collaborating Centres 3 Analysis and sequencing of variola virus genomes 3 Biosecurity and physical security of WHO collaborating laboratories 4 Smallpox vaccine stocks and production 4 Deliberate release of smallpox virus 4 Survey of WHO Member States latest position on destruction of variola virus 4 Recommendations 5 List of Participants 6 Page i REPORT OF THE MEETING OF THE AD HOC COMMITTEE ON ORTHOPOXVIRUS INFECTIONS Geneva, Switzerland 14-15 January 1999 Introduction Dr Lindsay Martinez, Director, Communicable Disease Surveillance and Response (CSR), welcomed participants and opened the meeting on behalf of the Director-General of WHO, Dr G.H. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus. -

A Tale of Two Viruses: Coinfections of Monkeypox and Varicella Zoster Virus in the Democratic Republic of Congo
Am. J. Trop. Med. Hyg., 104(2), 2021, pp. 604–611 doi:10.4269/ajtmh.20-0589 Copyright © 2021 by The American Society of Tropical Medicine and Hygiene A Tale of Two Viruses: Coinfections of Monkeypox and Varicella Zoster Virus in the Democratic Republic of Congo Christine M. Hughes,1* Lindy Liu,2,3 Whitni B. Davidson,1 Kay W. Radford,4 Kimberly Wilkins,1 Benjamin Monroe,1 Maureen G. Metcalfe,3 Toutou Likafi,5 Robert Shongo Lushima,6 Joelle Kabamba,7 Beatrice Nguete,5 Jean Malekani,8 Elisabeth Pukuta,9 Stomy Karhemere,9 Jean-Jacques Muyembe Tamfum,9 Emile Okitolonda Wemakoy,5 Mary G. Reynolds,1 D. Scott Schmid,4 and Andrea M. McCollum1 1Poxvirus and Rabies Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 2Bacterial Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 3Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 4Viral Vaccine Preventable Diseases Branch, Division of Viral Diseases, National Center for Immunizations and Respiratory Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 5Kinshasa School of Public Health, Kinshasa, Democratic Republic of Congo; 6Ministry of Health, Kinshasa, Democratic Republic of Congo; 7U.S. Centers for Disease Control and Prevention, Kinshasa, Democratic Republic of Congo; 8Department of Biology, University of Kinshasa, Kinshasa, Democratic Republic of Congo; 9Institut National de Recherche Biomedicale, ´ Kinshasa, Democratic Republic of Congo Abstract. -

Fact Sheet: Basic Information About Monkeypox
MONKEYPOX FACT SHEET Basic Information about Monkeypox Monkeypox: An Emerging Infectious Disease in North America Monkeypox is a rare viral disease that is found mostly in the rainforest countries of central and west Africa. The disease is called “monkeypox” because it was first discovered in laboratory monkeys in 1958. Blood tests of animals in Africa later found evidence of monkeypox infection in various rodent species. The virus that causes monkeypox was recovered from an African squirrel, which may be the natural host. Laboratory studies showed that the virus could also infect rats, mice, and rabbits. In 1970, monkeypox was identified as the cause of a rash illness in humans in remote African locations. In early June 2003, monkeypox was reported among several residents in the United States who became ill after having contact with sick pet prairie dogs. This is the first evidence of community-acquired monkeypox in the United States. Cause of Monkeypox The disease is caused by Monkeypox virus, which belongs to the orthopoxvirus group of viruses. Other orthopoxviruses that can cause infection in humans include variola (smallpox), vaccinia (used in smallpox vaccine), and cowpox viruses. Signs and Symptoms In humans, the signs and symptoms of monkeypox are similar to those of smallpox, but usually milder. Unlike smallpox, monkeypox causes swollen lymph nodes. The incubation period for monkeypox is about 12 days.The illness begins with fever, headache, muscle aches, backache, swollen lymph nodes, a general feeling of discomfort, and exhaustion. Within 1 to 3 days (sometimes longer) after onset of fever, the patient develops a papular rash (i.e., raised bumps), often first on the face but sometimes initially on other parts of the body. -

Universidad Autónoma De Madrid
UNIVERSIDAD AUTÓNOMA DE MADRID FACULTAD DE MEDICINA Departamento de Medicina Interna INVESTIGACIÓN DE LA ASOCIACIÓN ENTRE MANIFESTACIONES CUTÁNEAS E INFECCIÓN POR PARVOVIRUS B19 TESIS DOCTORAL Carlos Santonja Garriga Servicio de Anatomía Patológica Fundación Jiménez Díaz, Universidad Autónoma Madrid, 2017 DIRIGIDA POR el Doctor LUIS REQUENA CABALLERO AGRADECIMIENTOS A los Dres Luis Requena y Heinz Kutzner, por su inagotable generosidad A mi padre. Introducción General a) Presentación de los trabajos Los trabajos compendiados que constituyen el cuerpo de la presente tesis están relacionados con la infección por el Parvovirus humano B19 (B19V), los cuadros dermatopatológicos ocasionados por la misma y la hipótesis de que la detección aislada en biopsias cutáneas de ácido desoxirribonucleico (ADN) de B19V no constituye necesariamente prueba de relación causal entre aquella y estos, pues el virus es capaz de permanecer en los tejidos mucho tiempo después de ocurrida la infección. Tal relación debe basarse en determinaciones analíticas (seroconversión, presencia de ADN vírico en sangre) o confirmación de la presencia de material del virión en los tejidos, como la demostración de proteína vírica (Viral Protein 2, VP2) en el endotelio de vasos dérmicos. Esta última apoya la tesis de que el virus penetra en células generalmente poco permisivas valiéndose de un mecanismo de estímulo mediado por anticuerpos, en el curso de la primera fase de la infección. En el primero de los trabajos (Immunohistochemistry in the Diagnosis of Cutaneous Viral Infections) se detalla la aportación de la inmunohistoquímica al diagnóstico de infección reciente por B19V, en el marco de un estudio global de infecciones víricas de la piel. -

Here, There, and Everywhere: the Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses
viruses Review Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses Natalia Ingrid Oliveira Silva, Jaqueline Silva de Oliveira, Erna Geessien Kroon , Giliane de Souza Trindade and Betânia Paiva Drumond * Laboratório de Vírus, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais: Belo Horizonte, Minas Gerais 31270-901, Brazil; [email protected] (N.I.O.S.); [email protected] (J.S.d.O.); [email protected] (E.G.K.); [email protected] (G.d.S.T.) * Correspondence: [email protected] Abstract: The global emergence of zoonotic viruses, including poxviruses, poses one of the greatest threats to human and animal health. Forty years after the eradication of smallpox, emerging zoonotic orthopoxviruses, such as monkeypox, cowpox, and vaccinia viruses continue to infect humans as well as wild and domestic animals. Currently, the geographical distribution of poxviruses in a broad range of hosts worldwide raises concerns regarding the possibility of outbreaks or viral dissemination to new geographical regions. Here, we review the global host ranges and current epidemiological understanding of zoonotic orthopoxviruses while focusing on orthopoxviruses with epidemic potential, including monkeypox, cowpox, and vaccinia viruses. Keywords: Orthopoxvirus; Poxviridae; zoonosis; Monkeypox virus; Cowpox virus; Vaccinia virus; host range; wild and domestic animals; emergent viruses; outbreak Citation: Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range 1. Poxvirus and Emerging Diseases and Geographic Distribution of Zoonotic diseases, defined as diseases or infections that are naturally transmissible Zoonotic Orthopoxviruses. Viruses from vertebrate animals to humans, represent a significant threat to global health [1]. -

Discovery of Antivirals Against Smallpox
Discovery of antivirals against smallpox Stephen C. Harrisona,b, Bruce Albertsc, Ellie Ehrenfeldd, Lynn Enquiste, Harvey Finebergf, Steven L. McKnightg, Bernard Mossh, Michael O’Donnelli, Hidde Ploeghj, Sandra L. Schmidk, K. Peter Walterl, and Julie Theriotm aHarvard Medical School, Howard Hughes Medical Institute, Seeley Mudd Building, Room 130, 250 Longwood Avenue, Boston, MA 02115; cNational Academy of Sciences, 2101 Constitution Avenue, NW, Washington, DC 20418; dLaboratory of Infectious Disease, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 50, Room 6120, 50 South Drive, Bethesda, MD 20892; ePrinceton University, 314 Schultz Laboratory, Washington Road, Princeton, NJ 08544; fInstitute of Medicine, 2101 Constitution Avenue, NW, Washington, DC 20418; gDepartment of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; hLaboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 4, Room 229, 4 Center Drive, Bethesda, MD 20892; iLaboratory of DNA Replication, The Rockefeller University, Howard Hughes Medical Institute, 1230 York Avenue, New York, NY 10021; jDepartment of Pathology, Harvard Medical School, NRB, 77 Avenue Louis Pasteur, Boston, MA 02115; kDepartment of Cell Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037; lDepartment of Biochemistry and Biophysics, University of California School of Medicine, Howard Hughes Medical Institute, Box 0448, HSE 1001, San Francisco, CA 94143; and mDepartment of Biochemistry, Stanford University School of Medicine, Stanford, CA 94305 Contributed by Stephen C. Harrison, May 21, 2004 mallpox, a devastating infectious Whatever the likelihood of covertly dopoxviruses has a restricted and spe- disease dreaded throughout much held variola virus stocks, an intentional cific host array (Table 2). -
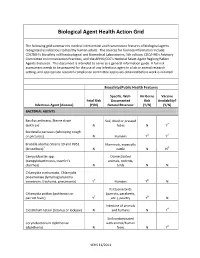
Biological Agent Health Action Grid
Biological Agent Health Action Grid The following grid summarizes medical intervention and transmission features of biological agents recognized as infectious to healthy human adults. The sources for foonote information include: CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories, 5th edition; CDC/HHS’s Advisory Committee on Immunization Practices; and the APHIS/CDC’s National Select Agent Registry/Select Agents Exclusion. This document is intended to serve as a general information guide. A full risk assessment needs to be prepared for the use of any infectious agent in a lab or animal research setting, and appropriate research compliance committee approvals obtained before work is initiated. Biosafety/Public Health Features Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) BACTERIAL AGENTS Bacillus anthracis, Sterne strain Soil, dried or pressed (anthrax)1 N hides N Y2 Bordetella pertussis (whooping cough or pertussis) N Humans Y3 Y4 Brucella abortus Strains 19 and RB51 Mammals, especially (brucellosis)5 N cattle N N6 Campylobacter spp. Domesticated (campylobacteriosis, traveler's animals, rodents, diarrhea) N birds N N Chlamydia trachomatis, Chlamydia pneumoniae (lymphogranuloma venereum, trachoma, pneumonia) Y7 Humans Y8 N Psittacine birds Chlamydia psittaci (psittacosis or (parrots, parakeets, parrott fever) Y7 etc.), poultry Y8 N Intestine of animals Clostridium tetani (tetanus or lockjaw) N and humans N Y4 Soil contaminated Corynebacterium diphtheriae with animal/human (diphtheria) N feces N Y4 VEHS 11/2011 Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) Francisella tularensis, subspecies Wild animals, esp. -

BMBL) Quickly Became the Cornerstone of Biosafety Practice and Policy in the United States Upon First Publication in 1984
Biosafety in Microbiological and Biomedical Laboratories 5th Edition U.S. Department of Health and Human Services Public Health Service Centers for Disease Control and Prevention National Institutes of Health HHS Publication No. (CDC) 21-1112 Revised December 2009 Foreword Biosafety in Microbiological and Biomedical Laboratories (BMBL) quickly became the cornerstone of biosafety practice and policy in the United States upon first publication in 1984. Historically, the information in this publication has been advisory is nature even though legislation and regulation, in some circumstances, have overtaken it and made compliance with the guidance provided mandatory. We wish to emphasize that the 5th edition of the BMBL remains an advisory document recommending best practices for the safe conduct of work in biomedical and clinical laboratories from a biosafety perspective, and is not intended as a regulatory document though we recognize that it will be used that way by some. This edition of the BMBL includes additional sections, expanded sections on the principles and practices of biosafety and risk assessment; and revised agent summary statements and appendices. We worked to harmonize the recommendations included in this edition with guidance issued and regulations promulgated by other federal agencies. Wherever possible, we clarified both the language and intent of the information provided. The events of September 11, 2001, and the anthrax attacks in October of that year re-shaped and changed, forever, the way we manage and conduct work -

Aerosol Transmissible Disease Biosafety Plan
AEROSOL TRANSMISSIBLE DISEASE BIOSAFETY PLAN Principal Investigator: Department: Date: I. EXPOSURE DETERMINATION: A. The following organisms and viruses are covered. We recognize that there is a high degree of variability in infectivity of these organisms and viruses. Check √ the organism(s) or viruses used in your laboratory: Adenovirus Helicobacter pylori4 Parvovirus B19 Arboviruses1 Hemorrhagic fever viruses5 Prions8 Arenaviruses2 Hendra virus Rabies virus9 Bacillus anthracis3 Hepatitis B Virus Retroviruses10 Blastomyces dermatitidis Hepatitis C Virus Rickettsia akari Bordetella pertussis Hepatitis D virus Rickettsia australis Brucella abortus Herpes Simplex Virus 1 Rickettsia conorii Brucella canis Herpes Simplex Virus 2 Rickettsia japonicum Brucella maris Herpesvirus simiae (B-virus) Rickettsia prowazekii Brucella melitensis Histoplasma capsulatum Rickettsia rickettsii Brucella suis Human Herpesvirus 6A Rickettsia siberica Burkholderia mallei Human Herpesvirus 6B Rickettsia typhi Burkholderia pseudomallei Human Herpesvirus 7 Rickettsia tsutsuagmushi Chlamydia pneumoniae Human Herpesvirus 8 Rift Valley fever virus Chlamydia psittaci Influenza Viruses6 Rubella Virus Chlamydia trachomatis Junin virus Sabia virus Clostridium botulinum Kyasanur forest disease virus Salmonella species Coccidioides immitis Lassa fever virus Salmonella typhi Coccidioides posadasii Legionella pneumophila SARS coronavirus Corynebacterium diphtheriae Lymphocytic Choriomeningitis Virus Shigella species Coxiella burnetti Machupo virus Streptococcus species, group -

Monkeypox Virus Liberia, and the US (Ex-Ghana)
APPENDIX 2 Monkeypox Virus Liberia, and the US (ex-Ghana). The West African clade is less virulent than the Congo Basin clade. Disease Agent: Common Human Exposure Routes: • Monkeypox virus (MPV) • Respiratory, percutaneous, and permucosal expo- Disease Agent Characteristics: sures to infected monkeys, zoo animals, prairie dogs, and humans • Family: Poxviridae; Subfamily: Chordopoxvirinae; Genus: Orthopoxvirus Likelihood of Secondary Transmission: • Virion morphology and size: Enveloped, slightly pleomorphic; dumbbell-shaped core with lateral • Direct contact with body fluids, respiratory droplets, bodies; 140-260 nm in diameter by 220-450 nm in or with virus-contaminated objects, such as bedding length or clothing • Nucleic acid: linear, double-stranded DNA virus; • Period of human-to-human transmission is during genome length: ~197 kb in length bp the first week of the rash. • Physicochemical properties: Resistant to common • Longest chain of documented human-to-human phenolic disinfectants; inactivated with polar lipo- transmission was five generations (four serial philic solvents, such as chloroform, and at low pH. transmissions). Complete inactivation of the closely related vaccinia At-Risk Populations: virus occurs in 2-3 hours at 60°C or within minutes following exposure to 20 nM caprylate at 22°C; • Very low in the US, based on animal import controls however, MPV is more resistant than vaccinia to established in 2003 solvent-detergent treatment. • In Africa, people coming in contact with infected animals Disease Name: Vector and Reservoir Involved: • Monkeypox • Reservoir is African rodents Priority Level: Blood Phase: • Scientific/Epidemiologic evidence regarding blood safety: Theoretical • In an outbreak in the Republic of Congo, one out of • Public perception and/or regulatory concern regard- five specimens was positive after 21 days. -

B Directive 2000/54/Ec of the European
02000L0054 — EN — 24.06.2020 — 002.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B DIRECTIVE 2000/54/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC) (OJ L 262, 17.10.2000, p. 21) Amended by: Official Journal No page date ►M1 Commission Directive (EU) 2019/1833 of 24 October 2019 L 279 54 31.10.2019 ►M2 Commission Directive (EU) 2020/739 of 3 June 2020 L 175 11 4.6.2020 02000L0054 — EN — 24.06.2020 — 002.001 — 2 ▼B DIRECTIVE 2000/54/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC) CHAPTER I GENERAL PROVISIONS Article 1 Objective 1. This Directive has as its aim the protection of workers against risks to their health and safety, including the prevention of such risks, arising or likely to arise from exposure to biological agents at work.