Ionization Mechanism of Positive-Ion Direct Analysis in Real Time: a Transient Microenvironment Concept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactivity and Functionalization of Naphthalene and Anthracene Complexes of {Tpw(NO)(Pme3)}
Reactivity and Functionalization of Naphthalene and Anthracene Complexes of {TpW(NO)(PMe3)} Laura Jessica Strausberg Baltimore, Maryland B.A., Hollins University, 2008 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Chemistry University of Virginia July, 2013 ii Abstract Chapter 1 introduces the organic chemistry of aromatic hydrocarbons, with attention paid to regiochemical outcomes of organic reactions. The binding of naphthalene and anthracene to metal complexes is discussed, along with organic transformations they undergo as a result of their complexation. The previous work on osmium and rhenium complexes of naphthalene from the Harman group is explored. Finally, some spectroscopic techniques for exploring the chemistry of {TpW(NO)(PMe3)} complexes of naphthalene and anthracene are introduced. Chapter 2 discusses the highly distorted allyl complexes formed from {TpW(NO)(PMe3)} and the exploration of their origin. Attempts at stereoselectively deprotonating these cationic complexes is also discussed. 2 Chapter 3 describes our study of TpW(NO)(PMe3)(3,4-η -naphthalene)’s ability to undergo a Diels-Alder reaction with N-methylmaleimide. A solvent study suggested that this reaction proceeds by a concerted mechanism. To probe the mechanism further, we synthesized a series of methylated and methoxylated naphthalene complexes and measured their rates of reaction with N-methylmaleimide compared to the parent complex. We found that 1- substitution on the naphthalene increased the rate of cycloaddition, even if the substituent was in the unbound ring, while 2-substitution slowed the reaction rate when in the bound ring. This information is consistent with a concerted mechanism, as a 2-substituted product would be less able to isomerize to form the active isomer for the cycloaddition to occur. -

Chapter 16 Liquid and Solids
Homework #2 Chapter 16 Liquid and Solids 7. Vapor Pressure: The pressure exerted by the vapor of a liquid when the vapor and the liquid are in dynamic equilibrium. The vapor pressure reflects the fact that within a system there is a distribution of energies that molecules can have, therefore, some molecules will have enough energy to overcome the intermolecular forces and enter into the gas phase. All liquids have some vapor pressure. The stronger the intermolecular forces the smaller the vapor pressure. All solids also have a vapor pressure. This is why if you leave ice in the freezer for a long time it “disappears.” The vapor pressure of solids is less than the vapor pressure of liquids. As the temperature increases the molecules have more energy, therefore, more molecules can escape into the gas phase (vapor pressure increases). When the vapor pressure is equal to the atmospheric pressure the solution boils. 9. a) Surface Tension As the intermolecular forces increase (↑), surface tension increases (↑). b) Viscosity As the intermolecular forces increase (↑), the viscosity increases (↑). c) Melting Point As the intermolecular forces increase (↑), the melting point increases (↑). d) Boiling Point As the intermolecular forces increase (↑), the boiling point increases (↑). e) Vapor Pressure As the intermolecular forces increase (↑), the vapor pressure decreases (↓). 11. Intermolecular Forces: The forces of attraction/repulsion between molecules. Intramolecular Forces: The forces of attraction/repulsion within a molecule. Intramolecular forces are stronger the intermolecular forces. Types of intermolecular forces: Dipole-Dipole Forces: The interaction between two electric dipoles in different molecules. Hydrogen Bonding: The attraction between a hydrogen atom (that is bonded to an O, N, or F atom) and an O, N, or F atom in a neighboring molecule. -

Contents • Introduction to Proteomics and Mass Spectrometry
Contents • Introduction to Proteomics and Mass spectrometry - What we need to know in our quest to explain life - Fundamental things you need to know about mass spectrometry • Interfaces and ion sources - Electrospray ionization (ESI) - conventional and nanospray - Heated nebulizer atmospheric pressure chemical ionization - Matrix assisted laser desorption • Types of MS analyzers - Magnetic sector - Quadrupole - Time-of-flight - Hybrid - Ion trap In the next step in our quest to explain what is life The human genome project has largely been completed and many other genomes are surrendering to the gene sequencers. However, all this knowledge does not give us the information that is needed to explain how living cells work. To do that, we need to study proteins. In 2002, mass spectrometry has developed to the point where it has the capacity to obtain the "exact" molecular weight of many macromolecules. At the present time, this includes proteins up to 150,000 Da. Proteins of higher molecular weights (up to 500,000 Da) can also be studied by mass spectrometry, but with less accuracy. The paradigm for sequencing of peptides and identification of proteins has changed – because of the availability of the human genome database, peptides can be identified merely by their masses or by partial sequence information, often in minutes, not hours. This new capacity is shifting the emphasis of biomedical research back to the functional aspects of cell biochemistry, the expression of particular sets of genes and their gene products, the proteins of the cell. These are the new goals of the biological scientist: o to know which proteins are expressed in each cell, preferably one cell at a time o to know how these proteins are modified, information that cannot necessarily be deduced from the nucleotide sequence of individual genes. -

WHO Guidelines for Indoor Air Quality : Selected Pollutants
WHO GUIDELINES FOR INDOOR AIR QUALITY WHO GUIDELINES FOR INDOOR AIR QUALITY: WHO GUIDELINES FOR INDOOR AIR QUALITY: This book presents WHO guidelines for the protection of pub- lic health from risks due to a number of chemicals commonly present in indoor air. The substances considered in this review, i.e. benzene, carbon monoxide, formaldehyde, naphthalene, nitrogen dioxide, polycyclic aromatic hydrocarbons (especially benzo[a]pyrene), radon, trichloroethylene and tetrachloroethyl- ene, have indoor sources, are known in respect of their hazard- ousness to health and are often found indoors in concentrations of health concern. The guidelines are targeted at public health professionals involved in preventing health risks of environmen- SELECTED CHEMICALS SELECTED tal exposures, as well as specialists and authorities involved in the design and use of buildings, indoor materials and products. POLLUTANTS They provide a scientific basis for legally enforceable standards. World Health Organization Regional Offi ce for Europe Scherfi gsvej 8, DK-2100 Copenhagen Ø, Denmark Tel.: +45 39 17 17 17. Fax: +45 39 17 18 18 E-mail: [email protected] Web site: www.euro.who.int WHO guidelines for indoor air quality: selected pollutants The WHO European Centre for Environment and Health, Bonn Office, WHO Regional Office for Europe coordinated the development of these WHO guidelines. Keywords AIR POLLUTION, INDOOR - prevention and control AIR POLLUTANTS - adverse effects ORGANIC CHEMICALS ENVIRONMENTAL EXPOSURE - adverse effects GUIDELINES ISBN 978 92 890 0213 4 Address requests for publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Scherfigsvej 8 DK-2100 Copenhagen Ø, Denmark Alternatively, complete an online request form for documentation, health information, or for per- mission to quote or translate, on the Regional Office web site (http://www.euro.who.int/pubrequest). -
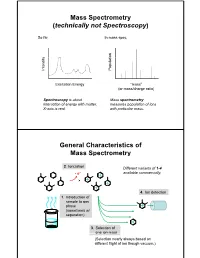
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

FID Vs PID: the Great Debate
5/5/2017 FID vs PID: The Great Debate 2017 Geotechnical, GeoEnvironmental, and Geophysical Technology Transfer April 11, 2017 Raleigh, NC Why is it Important? As consultants, we are expected to produce data that is Reliable Repeatable Representative Defensible We can only meet these criteria if we understand the instruments we use and their limitations 1 5/5/2017 PID=Photo Ionization Detector Non-destructive to the sample Responds to functional groups Can operate in non-oxygen atmosphere Does not respond to methane Affected by high humidity FID=Flame Ionization Detector Destructive to the sample Responds to carbon chain length Must have oxygen to operate Responds to methane Not affected by high humidity 2 5/5/2017 Combination FID/PID TVA 1000B Main Concepts Ionization Energy Minimum amount of energy required to remove an electron from an atom or molecule in a gaseous state Response Factors The response factor is a calculated number provided by the instrument manufacturer for each compound, which is used to calculate the actual concentration of said compound in relation to the calibration gas. 3 5/5/2017 Ionization Energy Basis for FID/PID operations and measurement Measurements are in electron volts (eV) Ionization in a PID Energy source for ionization with PID is an ultraviolet light Three UV lamp energies are used: 9.5 eV, 10.6 eV, and 11.7 eV The higher the lamp energy, the greater the number of chemicals that can be detected. Detection range of 0.1 to 10,000 ppm 4 5/5/2017 Ionization in a FID Energy source for ionization -

An Introduction to Mass Spectrometry
An Introduction to Mass Spectrometry by Scott E. Van Bramer Widener University Department of Chemistry One University Place Chester, PA 19013 [email protected] http://science.widener.edu/~svanbram revised: September 2, 1998 © Copyright 1997 TABLE OF CONTENTS INTRODUCTION ........................................................... 4 SAMPLE INTRODUCTION ....................................................5 Direct Vapor Inlet .......................................................5 Gas Chromatography.....................................................5 Liquid Chromatography...................................................6 Direct Insertion Probe ....................................................6 Direct Ionization of Sample ................................................6 IONIZATION TECHNIQUES...................................................6 Electron Ionization .......................................................7 Chemical Ionization ..................................................... 9 Fast Atom Bombardment and Secondary Ion Mass Spectrometry .................10 Atmospheric Pressure Ionization and Electrospray Ionization ....................11 Matrix Assisted Laser Desorption/Ionization ................................ 13 Other Ionization Methods ................................................13 Self-Test #1 ...........................................................14 MASS ANALYZERS .........................................................14 Quadrupole ............................................................15 -

Toxicological Profile for Naphthalene, 1
NAPHTHALENE, 1-METHYLNAPHTHALENE, AND 2-METHYLNAPHTHALENE 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about naphthalene, 1-methylnaphthalene, and 2-methyl- naphthalene and the effects of exposure to these chemicals. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites are then placed on the National Priorities List (NPL) and are targeted for long-term federal clean-up activities. Naphthalene, 1-methylnaphthalene, and 2-methyl- naphthalene have been found in at least 654, 36, and 412, respectively, of the 1,662 current or former NPL sites. Although the total number of NPL sites evaluated for these substances is not known, the possibility exists that the number of sites at which naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene are found may increase in the future as more sites are evaluated. This information is important because these sites may be sources of exposure and exposure to these substances may harm you. When a substance is released either from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. Such a release does not always lead to exposure. You can be exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. If you are exposed to naphthalene, 1-methylnaphthalene, or 2-methylnaphthalene, many factors will determine whether you will be harmed. These factors include the dose (how much), the duration (how long), and how you come in contact with them. -

Methods of Ion Generation
Chem. Rev. 2001, 101, 361−375 361 Methods of Ion Generation Marvin L. Vestal PE Biosystems, Framingham, Massachusetts 01701 Received May 24, 2000 Contents I. Introduction 361 II. Atomic Ions 362 A. Thermal Ionization 362 B. Spark Source 362 C. Plasma Sources 362 D. Glow Discharge 362 E. Inductively Coupled Plasma (ICP) 363 III. Molecular Ions from Volatile Samples. 364 A. Electron Ionization (EI) 364 B. Chemical Ionization (CI) 365 C. Photoionization (PI) 367 D. Field Ionization (FI) 367 IV. Molecular Ions from Nonvolatile Samples 367 Marvin L. Vestal received his B.S. and M.S. degrees, 1958 and 1960, A. Spray Techniques 367 respectively, in Engineering Sciences from Purdue Univesity, Layfayette, IN. In 1975 he received his Ph.D. degree in Chemical Physics from the B. Electrospray 367 University of Utah, Salt Lake City. From 1958 to 1960 he was a Scientist C. Desorption from Surfaces 369 at Johnston Laboratories, Inc., in Layfayette, IN. From 1960 to 1967 he D. High-Energy Particle Impact 369 became Senior Scientist at Johnston Laboratories, Inc., in Baltimore, MD. E. Low-Energy Particle Impact 370 From 1960 to 1962 he was a Graduate Student in the Department of Physics at John Hopkins University. From 1967 to 1970 he was Vice F. Low-Energy Impact with Liquid Surfaces 371 President at Scientific Research Instruments, Corp. in Baltimore, MD. From G. Flow FAB 371 1970 to 1975 he was a Graduate Student and Research Instructor at the H. Laser Ionization−MALDI 371 University of Utah, Salt Lake City. From 1976 to 1981 he became I. -

Report on Carcinogens, Fourteenth Edition for Table of Contents, See Home Page
Report on Carcinogens, Fourteenth Edition For Table of Contents, see home page: http://ntp.niehs.nih.gov/go/roc Naphthalene comes from studies of workers in a coke plant, which found that con- centrations of naphthalene metabolites in the urine were significantly CAS No. 91-20-3 correlated with concentrations of naphthalene in personal air sam- ples (Bieniek 1994, 1997). The first step in the metabolism of naph- Reasonably anticipated to be a human carcinogen thalene is formation of naphthalene-1,2-oxide (as two stereo isomers, First listed in the Eleventh Report on Carcinogens (2004) 1R,2S-oxide and 1S,2R-oxide) through the action of cytochrome P450 enzymes in the presence of the coenzyme NADPH. These oxides are metabolized further by three pathways: (1) hydration by epoxide hy- drolases into dihydrodiols, (2) conjugation by glutathione transferases, and (3) spontaneous rearrangement into 1-naphthol and 2-naph- Carcinogenicity thol, which are converted to naphthoquinones (Chichester et al. 1994, Shultz et al. 1999). Naphthalene is excreted in the urine as the un- Naphthalene is reasonably anticipated to be a human carcinogen changed parent compound or as metabolites, including 1-naphthol, based on sufficient evidence from studies in experimental animals. 2-naphthol, naphthoquinones, dihydroxynaphthalenes, and conju- gated forms, including glutathione, cysteine, glucuronic acid, and Cancer Studies in Experimental Animals sulfate conjugates (NTP 2002). Exposure of rats to naphthalene by inhalation caused nasal tumors, The mechanism by which naphthalene causes cancer is unknown. which are rare in this species. Two types of nasal tumor were ob- A strong correlation has been observed between the rates of forma- served: olfactory epithelial neuroblastoma of the nose, which is a tion of the stereoisomer (1R,2S)-naphthalene oxide in various tissues highly malignant and extremely rare tumor of the lining of the nose, and the selective toxicity of naphthalene to these tissues, suggesting and respiratory epithelial adenoma, which also is rare (NTP 2000). -

Studies on the Adsorption of Naphthalene and Pyrene from Aqueous Medium Using Ripe Orange Peels As Adsorbent
GLOBAL JOURNAL OF PURE AND APPLIED SCIENCES VOL 16, NO. 1, 2010: 131-139 131 COPYRIGHT© BACHUDO SCIENCE CO. LTD PRINTED IN NIGERIA. ISSN 1118-057 STUDIES ON THE ADSORPTION OF NAPHTHALENE AND PYRENE FROM AQUEOUS MEDIUM USING RIPE ORANGE PEELS AS ADSORBENT C.N. OWABOR AND J. E. AUDU (Received 14, May 2009; Revision Accepted 23, October 2009) ABSTRACT The effectiveness of dried ground orange peels in adsorbing naphthalene and pyrene from an aqueous stream has been investigated in terms of variation in concentration, adsorbent dosage, agitation time and particle size. Experimental batch data was correlated by Freundlich and Langmuir isotherm models. The Freundlich isotherm best described the adsorption process as the adsorption data fitted well into the model. The adsorption capacity and energy of adsorption were obtained as 7.519mg/g and 0.0863mg -1, and 3.8168mg/g and 0.0334mg -1 for naphthalene and pyrene respectively. The adsorption from the aqueous solution was observed to be time dependent while equilibrium time was found to be 100 and 120 minutes for naphthalene and pyrene respectively. Adsorption increased with increase in adsorbent dosage and was maximum at between 5 to 7g for naphthalene and 6 to 8g for pyrene. The maximum adsorption was observed using a particle size of 2.0mm. The rate of adsorption using the first order rate expression by Lagergren for naphthalene and pyrene were 0.007 and 0.006 min -1 respectively. These results therefore suggest that naphthalene is more selectively adsorbed than pyrene using ripe orange peel as adsorbent. KEY WORDS: Naphthalene, Pyrene, Adsorption, Adsorbent, Ripe orange peels. -

Selective Chemical Ionization of Nitrogen and Sulfur Heterocycles in Petroleum Fractions by Ion Trap Mass Spectrometry
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Selective Chemical Ionization of Nitrogen and Sulfur Heterocycles in Petroleum Fractions by Ion Trap Mass Spectrometry C. S. Creaser*, F. Krokos, and K. E. O’Neill Shod of Chemical Sciences, University of East Anglia, Norwich, United Kingdom M. J. C. Smith and P. G. McDowell BP Research, Sunbury-on-Thames, Middlesex, United Kingdom A procedure is reported for the selective ammonia chemical ionization of some nitrogen and sulfur heterocycles in petroleum fractions using ion trap mass spectrometry (ITMS). The ion trap scan routine is designed to optimize the population of ammonium reagent ions and eject from the trap (by radio frequency/direct current isolation) electron ionization products formed during reagent ion formation prior to ionization of the sample. The ITMS procedure is compared with standard ion trap detector and conventional quadrupole ammonia chemi- cal ionization for the determination of nitrogen and sulfur heterocycles in gas oil and kerosine samples. Greatly enhanced selectivity is shown for the ITMS procedure by sup res- sion of competing charge-exchange processes. (1 Am Sot Mass Spectrum 1993, 4, 322-326 ‘; he removal of organic compounds containing reagent ion NH;, is formed via the reactions [5] nitrogen and sulfur is an important part of the T petroleum-refining process 111. Their presence in NH, ‘1 NH;’ (1) petroleum distillates results in the formation of envi- NH, f NH:‘+ NH; + NH; (2) ronmental pollutants (SO,, NO,) during combustion, and their reactivity can lead to catalyst poisoning Whereas NH: is unreactive toward a wide range of in refinery processes.