Electrochemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Next Generation Anodes for Lithium-Ion Batteries
NEXT GENE ATION ANODES Next Ge eratio A odes for Lithium-Io Batteries Seco d Quarter Progress Report 2018 De is Dees, Poi t-of-Co tact Argonne National Laboratory 9700 South Cass Avenue Argonne, IL 60439 Phone: (630) 252-7349 E-mail: [email protected] Bria Cu i gham, DOE-EERE-VTO Tech ology Ma ager U.S. Department of Energy, Battery R&D Phone: (202) 287-5686 E-mail: [email protected] Table of Co te ts Overview (page 2) 1. Research Facilities Support (page 5) CAMP Facility Support Activities (ANL) (page 5) Characterization and Optimization of Si Slurry by Zeta Potential (ORNL) (page 7) Thermodynamic Understanding and Abuse Performance (SNL) (page 9) Hydro/Solvothermal Synthesis and Scale-up of Silicon and Silicon-containing Nanoparticles (ANL) (page 10) 2. Characterization, Diagnostics, and Analysis (page 12) Spectroscopic Characterization of Cycled Si Electrodes: Understanding the Role of Binder (ORNL) (page 12) EQCM Studies of Silicon Anodes (ANL) (page 13) Effect of silicate thickness on the electrochemical performance of silicate-coated silicon nanoparticles (ANL) (page 15) Calendar-life versus Cycle-life aging of Lithium-ion Cells with Silicon-Graphite Composite Electrodes – Electrolyte Analysis (ANL) (page 17) 3. Materials Advancements (page 18) Continued Study of Lithiation Effect of the Poly(Acrylic Acid) Binders on the Silicon Anode of Lithium-Ion Batteries (ANL) (page 19) Probe the relationships between functional electrolytes structure and SEI property for Si materials (LBNL) (page 21) Silicon Surface Modification Using -

Elements of Electrochemistry
Page 1 of 8 Chem 201 Winter 2006 ELEM ENTS OF ELEC TROCHEMIS TRY I. Introduction A. A number of analytical techniques are based upon oxidation-reduction reactions. B. Examples of these techniques would include: 1. Determinations of Keq and oxidation-reduction midpoint potentials. 2. Determination of analytes by oxidation-reductions titrations. 3. Ion-specific electrodes (e.g., pH electrodes, etc.) 4. Gas-sensing probes. 5. Electrogravimetric analysis: oxidizing or reducing analytes to a known product and weighing the amount produced 6. Coulometric analysis: measuring the quantity of electrons required to reduce/oxidize an analyte II. Terminology A. Reduction: the gaining of electrons B. Oxidation: the loss of electrons C. Reducing agent (reductant): species that donates electrons to reduce another reagent. (The reducing agent get oxidized.) D. Oxidizing agent (oxidant): species that accepts electrons to oxidize another species. (The oxidizing agent gets reduced.) E. Oxidation-reduction reaction (redox reaction): a reaction in which electrons are transferred from one reactant to another. 1. For example, the reduction of cerium(IV) by iron(II): Ce4+ + Fe2+ ! Ce3+ + Fe3+ a. The reduction half-reaction is given by: Ce4+ + e- ! Ce3+ b. The oxidation half-reaction is given by: Fe2+ ! e- + Fe3+ 2. The half-reactions are the overall reaction broken down into oxidation and reduction steps. 3. Half-reactions cannot occur independently, but are used conceptually to simplify understanding and balancing the equations. III. Rules for Balancing Oxidation-Reduction Reactions A. Write out half-reaction "skeletons." Page 2 of 8 Chem 201 Winter 2006 + - B. Balance the half-reactions by adding H , OH or H2O as needed, maintaining electrical neutrality. -

All-Carbon Electrodes for Flexible Solar Cells
applied sciences Article All-Carbon Electrodes for Flexible Solar Cells Zexia Zhang 1,2,3 ID , Ruitao Lv 1,2,*, Yi Jia 4, Xin Gan 1,5 ID , Hongwei Zhu 1,2 and Feiyu Kang 1,5,* 1 State Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China; [email protected] (Z.Z.); [email protected] (X.G.); [email protected] (H.Z.) 2 Key Laboratory of Advanced Materials (MOE), School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China 3 School of Physics and Electronic Engineering, Xinjiang Normal University, Urumqi 830046, Xinjiang Province, China 4 Qian Xuesen Laboratory of Space Technology, China Academy of Space Technology, Beijing 100094, China; [email protected] 5 Graduate School at Shenzhen, Tsinghua University, Shenzhen 518055, Guangdong Province, China * Correspondences: [email protected] (R.L.); [email protected] (F.K.) Received: 16 December 2017; Accepted: 20 January 2018; Published: 23 January 2018 Abstract: Transparent electrodes based on carbon nanomaterials have recently emerged as new alternatives to indium tin oxide (ITO) or noble metal in organic photovoltaics (OPVs) due to their attractive advantages, such as long-term stability, environmental friendliness, high conductivity, and low cost. However, it is still a challenge to apply all-carbon electrodes in OPVs. Here, we report our efforts to develop all-carbon electrodes in organic solar cells fabricated with different carbon-based materials, including carbon nanotubes (CNTs) and graphene films synthesized by chemical vapor deposition (CVD). Flexible and semitransparent solar cells with all-carbon electrodes are successfully fabricated. -

A Review of Cathode and Anode Materials for Lithium-Ion Batteries
A Review of Cathode and Anode Materials for Lithium-Ion Batteries Yemeserach Mekonnen Aditya Sundararajan Arif I. Sarwat IEEE Student Member IEEE Student Member IEEE Member Department of Electrical & Department of Electrical & Department of Electrical & Computer Engineering Computer Engineering Computer Engineering Florida International University Florida International University Florida International University Email: [email protected] Email: [email protected] Email: [email protected] Abstract—Lithium ion batteries are one of the most technologies such as plug-in HEVs. For greater application use, commercially sought after energy storages today. Their batteries are usually expensive and heavy. Li-ion and Li- based application widely spans from Electric Vehicle (EV) to portable batteries show promising advantages in creating smaller, devices. Their lightness and high energy density makes them lighter and cheaper battery storage for such high-end commercially viable. More research is being conducted to better applications [18]. As a result, these batteries are widely used in select the materials for the anode and cathode parts of Lithium (Li) ion cell. This paper presents a comprehensive review of the common consumer electronics and account for higher sale existing and potential developments in the materials used for the worldwide [2]. Lithium, as the most electropositive element making of the best cathodes, anodes and electrolytes for the Li- and the lightest metal, is a unique element for the design of ion batteries such that maximum efficiency can be tapped. higher density energy storage systems. The discovery of Observed challenges in selecting the right set of materials is also different inorganic compounds that react with alkali metals in a described in detail. -

A Highly-Active, Stable and Low-Cost Platinum-Free Anode Catalyst Based on Runi for Hydroxide Exchange Membrane Fuel Cells
ARTICLE https://doi.org/10.1038/s41467-020-19413-5 OPEN A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells Yanrong Xue1,2,5, Lin Shi3,5, Xuerui Liu1,2, Jinjie Fang1,2, Xingdong Wang2, Brian P. Setzler3, Wei Zhu 2, ✉ ✉ Yushan Yan 1,3 & Zhongbin Zhuang 1,2,4 1234567890():,; The development of cost-effective hydroxide exchange membrane fuel cells is limited by the lack of high-performance and low-cost anode hydrogen oxidation reaction catalysts. Here we report a Pt-free catalyst Ru7Ni3/C, which exhibits excellent hydrogen oxidation reaction activity in both rotating disk electrode and membrane electrode assembly measurements. The hydrogen oxidation reaction mass activity and specific activity of Ru7Ni3/C, as measured in rotating disk experiments, is about 21 and 25 times that of Pt/C, and 3 and 5 times that of PtRu/C, respectively. The hydroxide exchange membrane fuel cell with Ru7Ni3/C anode can −2 −2 deliver a high peak power density of 2.03 W cm in H2/O2 and 1.23 W cm in H2/air (CO2-free) at 95 °C, surpassing that using PtRu/C anode catalyst, and good durability with less than 5% voltage loss over 100 h of operation. The weakened hydrogen binding of Ru by alloying with Ni and enhanced water adsorption by the presence of surface Ni oxides lead to the high hydrogen oxidation reaction activity of Ru7Ni3/C. By using the Ru7Ni3/C catalyst, the anode cost can be reduced by 85% of the current state-of-the-art PtRu/C, making it highly promising in economical hydroxide exchange membrane fuel cells. -

Galvanic Cell Notation • Half-Cell Notation • Types of Electrodes • Cell
Galvanic Cell Notation ¾Inactive (inert) electrodes – not involved in the electrode half-reaction (inert solid conductors; • Half-cell notation serve as a contact between the – Different phases are separated by vertical lines solution and the external el. circuit) 3+ 2+ – Species in the same phase are separated by Example: Pt electrode in Fe /Fe soln. commas Fe3+ + e- → Fe2+ (as reduction) • Types of electrodes Notation: Fe3+, Fe2+Pt(s) ¾Active electrodes – involved in the electrode ¾Electrodes involving metals and their half-reaction (most metal electrodes) slightly soluble salts Example: Zn2+/Zn metal electrode Example: Ag/AgCl electrode Zn(s) → Zn2+ + 2e- (as oxidation) AgCl(s) + e- → Ag(s) + Cl- (as reduction) Notation: Zn(s)Zn2+ Notation: Cl-AgCl(s)Ag(s) ¾Electrodes involving gases – a gas is bubbled Example: A combination of the Zn(s)Zn2+ and over an inert electrode Fe3+, Fe2+Pt(s) half-cells leads to: Example: H2 gas over Pt electrode + - H2(g) → 2H + 2e (as oxidation) + Notation: Pt(s)H2(g)H • Cell notation – The anode half-cell is written on the left of the cathode half-cell Zn(s) → Zn2+ + 2e- (anode, oxidation) + – The electrodes appear on the far left (anode) and Fe3+ + e- → Fe2+ (×2) (cathode, reduction) far right (cathode) of the notation Zn(s) + 2Fe3+ → Zn2+ + 2Fe2+ – Salt bridges are represented by double vertical lines ⇒ Zn(s)Zn2+ || Fe3+, Fe2+Pt(s) 1 + Example: A combination of the Pt(s)H2(g)H Example: Write the cell reaction and the cell and Cl-AgCl(s)Ag(s) half-cells leads to: notation for a cell consisting of a graphite cathode - 2+ Note: The immersed in an acidic solution of MnO4 and Mn 4+ reactants in the and a graphite anode immersed in a solution of Sn 2+ overall reaction are and Sn . -

Nickel-Metal Hydride Batteries: a New Generation of Secondary Storage
8 Nickel-Metal Hydride Batteries: A New Generation of Secondary Storage Brian Niece Literature Seminar September 23, 1993 Interest in secondary batteries suitable for use in electric vehicles has increased rapidly in the last decade, as restrictions on vehicle emissions in urban areas have become more stringent. Combustion of fossil fuels in large power plants and storage of the energy electrochemically is more efficient than direct combustion in internal combustion engines [1]. In addition, storage batteries can be charged from alternative sources of electricity, such as solar and hydroelectric plants. In order to be acceptable for use in an electric vehicle, a battery must meet a number of important criteria. It must have a high energy density to make the travel distance between recharges as long as possible. It must be capable of high power output throughout its dis charge cycle to provide the high current necessary to operate a vehicle, and it must be capa ble of especially high peak power for acceleration. The battery must also have a long cycle life as well as a long physical lifetime. It must not undergo rapid self discharge on standing. Finally, it must not be prohibitively expensive. Theoretical energy densities of secondary battery systems in common use are listed in Table 1. Most of these systems are not practical for electric vehicle use for a variety of rea sons. The lead-sulfuric acid battery cannot be sealed, and cannot be produced with a suffi ciently high energy density. The sodium-sulfur cell is undesirable because it contains metal lic sodium and operates at 300°C. -

Electrochemistry –An Oxidizing Agent Is a Species That Oxidizes Another Species; It Is Itself Reduced
Oxidation-Reduction Reactions Chapter 17 • Describing Oxidation-Reduction Reactions Electrochemistry –An oxidizing agent is a species that oxidizes another species; it is itself reduced. –A reducing agent is a species that reduces another species; it is itself oxidized. Loss of 2 e-1 oxidation reducing agent +2 +2 Fe( s) + Cu (aq) → Fe (aq) + Cu( s) oxidizing agent Gain of 2 e-1 reduction Skeleton Oxidation-Reduction Equations Electrochemistry ! Identify what species is being oxidized (this will be the “reducing agent”) ! Identify what species is being •The study of the interchange of reduced (this will be the “oxidizing agent”) chemical and electrical energy. ! What species result from the oxidation and reduction? ! Does the reaction occur in acidic or basic solution? 2+ - 3+ 2+ Fe (aq) + MnO4 (aq) 6 Fe (aq) + Mn (aq) Steps in Balancing Oxidation-Reduction Review of Terms Equations in Acidic solutions 1. Assign oxidation numbers to • oxidation-reduction (redox) each atom so that you know reaction: involves a transfer of what is oxidized and what is electrons from the reducing agent to reduced 2. Split the skeleton equation into the oxidizing agent. two half-reactions-one for the oxidation reaction (element • oxidation: loss of electrons increases in oxidation number) and one for the reduction (element decreases in oxidation • reduction: gain of electrons number) 2+ 3+ - 2+ Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) 1 3. Complete and balance each half reaction Galvanic Cell a. Balance all atoms except O and H 2+ 3+ - 2+ (Voltaic Cell) Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) b. -

Hydrogen Storage Alloys
www.aladdin-e.com Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: [email protected] Email EU: [email protected] Email Asia Pacific: [email protected] Hydrogen storage alloys Hydrogen storage alloys are important for a few voltage (with respect to the counter electrode) is electrochemical applications, especially in the applied to the metal/metal hydride electrode energy storage area. The basic of current collector, and electrons enter the metal electrochemical use of the hydrogen storage through the current collector to neutralize the alloy can be described as follows: when protons from the splitting of water that occurs at hydrogen enters the lattice of most transition the metal/electrolyte interface (Figure 1a). This metals, interstitial metal hydride (MH) is formed. electrochemical charging process is The electrons accompanying the hydrogen atoms characterized by the half reaction: form a metal-hydrogen band right below the Fermi level, which indicates that the interstitial (1) MH is metallic in nature. While protons in the interstitial MH hop between neighboring During discharge, protons in the MH leave the occupation sites by quantum mechanical surface and recombine with OH− in the alkaline tunneling, the electrons remain within a short electrolyte to form H2O, and charge neutrality distance (3–10 angstroms) of the protons to pushes the electrons out of the MH through the maintain local charge neutrality. Under the current collector, performing electrical work in the influence of an electric field, electrons and attached circuitry (Figure 1b). The protons will move in opposite directions. In an electrochemical discharge process is given by electrochemical environment, a voltage is applied the half reaction: to cause electrons to flow, and the charges are balanced out by moving conductive ions through (2) a highly alkaline aqueous electrolyte with good ionic conductivity. -

Battery Technologies for Small Scale Embeded Generation
Battery Technologies for Small Scale Embedded Generation. by Norman Jackson, South African Energy Storage Association (SAESA) Content Provider – Wikipedia et al Small Scale Embedded Generation - SSEG • SSEG is very much a local South African term for Distributed Generation under 10 Mega Watt. Internationally they refer to: Distributed generation, also distributed energy, on-site generation (OSG) or district/decentralized energy It is electrical generation and storage performed by a variety of small, grid- connected devices referred to as distributed energy resources (DER) Types of Energy storage: • Fossil fuel storage • Thermal • Electrochemical • Mechanical • Brick storage heater • Compressed air energy storage • Cryogenic energy storage (Battery Energy • Fireless locomotive • Liquid nitrogen engine Storage System, • Flywheel energy storage • Eutectic system BESS) • Gravitational potential energy • Ice storage air conditioning • Hydraulic accumulator • Molten salt storage • Flow battery • Pumped-storage • Phase-change material • Rechargeable hydroelectricity • Seasonal thermal energy battery • Electrical, electromagnetic storage • Capacitor • Solar pond • UltraBattery • Supercapacitor • Steam accumulator • Superconducting magnetic • Thermal energy energy storage (SMES, also storage (general) superconducting storage coil) • Chemical • Biological • Biofuels • Glycogen • Hydrated salts • Starch • Hydrogen storage • Hydrogen peroxide • Power to gas • Vanadium pentoxide History of the battery This was a stack of copper and zinc Italian plates, -

2020 Grid Energy Storage Technology Cost and Performance Assessment
Energy Storage Grand Challenge Cost and Performance Assessment 2020 December 2020 2020 Grid Energy Storage Technology Cost and Performance Assessment Kendall Mongird, Vilayanur Viswanathan, Jan Alam, Charlie Vartanian, Vincent Sprenkle*, Pacific Northwest National LaBoratory. Richard Baxter, Mustang Prairie Energy * [email protected] Technical Report Publication No. DOE/PA-0204 December 2020 Energy Storage Grand Challenge Cost and Performance Assessment 2020 December 2020 Disclaimer This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government. ii Energy Storage Grand Challenge Cost and Performance Assessment 2020 December 2020 Acronyms AC alternating current Ah ampere-hour BESS battery energy storage system BLS U.S. Bureau of Labor Statistics BMS battery management system BOP balance of plant BOS balance of system C&C controls & communication C&I civil and infrastructure CAES compressed-air energy -
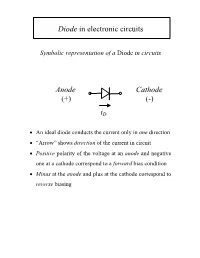
Diode in Electronic Circuits Anode Cathode (+) (-) Id
Diode in electronic circuits Symbolic representation of a Diode in circuits Anode Cathode (+) (-) iD • An ideal diode conducts the current only in one direction • “Arrow” shows direction of the current in circuit • Positive polarity of the voltage at an anode and negative one at a cathode correspond to a forward bias condition • Minus at the anode and plus at the cathode correspond to reverse biasing Diode as a non-linear resistor Current-Voltage (I-V) Characteristics I-V of a LINEAR RESISTOR i(A) R=v/i v(V) I-V of an IDEALIZED DIODE i(A) R → 0 v(V) R →∞ I-V Characteristic of an Ideal Diode qV /kT JJ=−S ()e 1 i(A) ~ exp(qV/kT) v(V) • At room temperature T=300K the thermal voltage kT/q = 26 meV • For a Si diode the typical value of the saturation current -10 2 JS ~ 10 A/cm JJ≈ eqV /kT For a high forward bias V >> 26 mV: S • The forward current shows an exponential dependence For a high reverse bias V <0, V< < -26 mV: J ≈ −J S • Reverse current of diodes is quite small I-V Characteristic of a Diode in semilogarithmic scale J(A/cm2) non-exp 102 increase 10-2 saturation slope -1 region 10-6 q/nkT~ 20-40 V |J | S 10-10 -10 10 20 30 qV/kT • The slop in logarithmic scale can be used to define the non-ideality factor n • The intersection of lgI-V characteristic with vertical axis gives the value of saturation current JS • The current increase differs from exponential at high forward bias voltage I-V Characteristic of a Real Diode A Silicon (Si) Diode i(A) v(V) 0.7 V • The typical voltage drop across a Si diode at forward bias is 0.7V A Germanium