BARK ANATOMY and INTERCELLULAR CANALS in the STEM of DELARBREA PARADOXA (ARALIACEAE) by M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bark Anatomy and Cell Size Variation in Quercus Faginea
Turkish Journal of Botany Turk J Bot (2013) 37: 561-570 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1201-54 Bark anatomy and cell size variation in Quercus faginea 1,2, 2 2 2 Teresa QUILHÓ *, Vicelina SOUSA , Fatima TAVARES , Helena PEREIRA 1 Centre of Forests and Forest Products, Tropical Research Institute, Tapada da Ajuda, 1347-017 Lisbon, Portugal 2 Centre of Forestry Research, School of Agronomy, Technical University of Lisbon, Tapada da Ajuda, 1349-017 Lisbon, Portugal Received: 30.01.2012 Accepted: 27.09.2012 Published Online: 15.05.2013 Printed: 30.05.2013 Abstract: The bark structure of Quercus faginea Lam. in trees 30–60 years old grown in Portugal is described. The rhytidome consists of 3–5 sequential periderms alternating with secondary phloem. The phellem is composed of 2–5 layers of cells with thin suberised walls and narrow (1–3 seriate) tangential band of lignified thick-walled cells. The phelloderm is thin (2–3 seriate). Secondary phloem is formed by a few tangential bands of fibres alternating with bands of sieve elements and axial parenchyma. Formation of conspicuous sclereids and the dilatation growth (proliferation and enlargement of parenchyma cells) affect the bark structure. Fused phloem rays give rise to broad rays. Crystals and druses were mostly seen in dilated axial parenchyma cells. Bark thickness, sieve tube element length, and secondary phloem fibre wall thickness decreased with tree height. The sieve tube width did not follow any regular trend. In general, the fibre length had a small increase toward breast height, followed by a decrease towards the top. -

Department of the Interior Fish and Wildlife Service
Thursday, February 27, 2003 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Final Designation or Nondesignation of Critical Habitat for 95 Plant Species From the Islands of Kauai and Niihau, HI; Final Rule VerDate Jan<31>2003 13:12 Feb 26, 2003 Jkt 200001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\27FER2.SGM 27FER2 9116 Federal Register / Vol. 68, No. 39 / Thursday, February 27, 2003 / Rules and Regulations DEPARTMENT OF THE INTERIOR units designated for the 83 species. This FOR FURTHER INFORMATION CONTACT: Paul critical habitat designation requires the Henson, Field Supervisor, Pacific Fish and Wildlife Service Service to consult under section 7 of the Islands Office at the above address Act with regard to actions carried out, (telephone 808/541–3441; facsimile 50 CFR Part 17 funded, or authorized by a Federal 808/541–3470). agency. Section 4 of the Act requires us SUPPLEMENTARY INFORMATION: RIN 1018–AG71 to consider economic and other relevant impacts when specifying any particular Background Endangered and Threatened Wildlife area as critical habitat. This rule also and Plants; Final Designation or In the Lists of Endangered and determines that designating critical Nondesignation of Critical Habitat for Threatened Plants (50 CFR 17.12), there habitat would not be prudent for seven 95 Plant Species From the Islands of are 95 plant species that, at the time of species. We solicited data and Kauai and Niihau, HI listing, were reported from the islands comments from the public on all aspects of Kauai and/or Niihau (Table 1). -

Recovery Plan for Tyoj5llllt . I-Bland Plants
Recovery Plan for tYOJ5llllt. i-bland Plants RECOVERY PLAN FOR MULTI-ISLAND PLANTS Published by U.S. Fish and Wildlife Service Portland, Oregon Approved: Date: / / As the Nation’s principal conservation agency, the Department of the Interior has responsibility for most ofour nationally owned public lands and natural resources. This includes fostering the wisest use ofour land and water resources, protecting our fish and wildlife, preserving the environmental and cultural values ofour national parks and historical places, and providing for the enjoyment of life through outdoor recreation. The Department assesses our energy and mineral resources and works to assure that their development is in the best interests ofall our people. The Department also has a major responsibility for American Indian reservation communities and for people who live in island Territories under U.S. administration. DISCLAIMER PAGE Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance ofrecovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Costs indicated for task implementation and/or time for achievement ofrecovery are only estimates and are subject to change. Recovery plans do not necessarily represent the views nor the official positions or approval ofany individuals or agencies involved in the plan formulation, otherthan the U.S. Fish and Wildlife Service. They represent the official position ofthe U.S. -

Keauhou Bird Conservation Center
KEAUHOU BIRD CONSERVATION CENTER Discovery Forest Restoration Project PO Box 2037 Kamuela, HI 96743 Tel +1 808 776 9900 Fax +1 808 776 9901 Responsible Forester: Nicholas Koch [email protected] +1 808 319 2372 (direct) Table of Contents 1. CLIENT AND PROPERTY INFORMATION .................................................................... 4 1.1. Client ................................................................................................................................................ 4 1.2. Consultant ....................................................................................................................................... 4 2. Executive Summary .................................................................................................. 5 3. Introduction ............................................................................................................. 6 3.1. Site description ............................................................................................................................... 6 3.1.1. Parcel and location .................................................................................................................. 6 3.1.2. Site History ................................................................................................................................ 6 3.2. Plant ecosystems ............................................................................................................................ 6 3.2.1. Hydrology ................................................................................................................................ -

Weed Risk Assessment for Pistacia Chinensis Bunge (Anacardiaceae)
Weed Risk Assessment for Pistacia United States chinensis Bunge (Anacardiaceae) – Department of Agriculture Chinese pistache Animal and Plant Health Inspection Service November 27, 2012 Version 1 Pistacia chinensis (source: D. Boufford, efloras.com) Agency Contact: Plant Epidemiology and Risk Analysis Laboratory Center for Plant Health Science and Technology Plant Protection and Quarantine Animal and Plant Health Inspection Service United States Department of Agriculture 1730 Varsity Drive, Suite 300 Raleigh, NC 27606 Weed Risk Assessment for Pistacia chinensis Introduction Plant Protection and Quarantine (PPQ) regulates noxious weeds under the authority of the Plant Protection Act (7 U.S.C. § 7701-7786, 2000) and the Federal Seed Act (7 U.S.C. § 1581-1610, 1939). A noxious weed is defined as “any plant or plant product that can directly or indirectly injure or cause damage to crops (including nursery stock or plant products), livestock, poultry, or other interests of agriculture, irrigation, navigation, the natural resources of the United States, the public health, or the environment” (7 U.S.C. § 7701-7786, 2000). We use weed risk assessment (WRA)—specifically, the PPQ WRA model (Koop et al., 2012)—to evaluate the risk potential of plants, including those newly detected in the United States, those proposed for import, and those emerging as weeds elsewhere in the world. Because the PPQ WRA model is geographically and climatically neutral, it can be used to evaluate the baseline invasive/weed potential of any plant species for the entire United States or for any area within it. As part of this analysis, we use a stochastic simulation to evaluate how much the uncertainty associated with the analysis affects the model outcomes. -
![Vascular Plants of Williamson County Rhus Aromatica − SKUNKBRUSH, FRAGRANT SUMAC [Anacardiaceae]](https://docslib.b-cdn.net/cover/8595/vascular-plants-of-williamson-county-rhus-aromatica-skunkbrush-fragrant-sumac-anacardiaceae-408595.webp)
Vascular Plants of Williamson County Rhus Aromatica − SKUNKBRUSH, FRAGRANT SUMAC [Anacardiaceae]
Vascular Plants of Williamson County Rhus aromatica − SKUNKBRUSH, FRAGRANT SUMAC [Anacardiaceae] Rhus aromatica Aiton (includes varieties), SKUNKBRUSH, FRAGRANT SUMAC. Shrub, winter-deciduous, clump-forming, with long shoots and short lateral and spur shoots, 50– 200 cm tall; shoots short-tomentose, strongly aromatic like wintergreen (Gaultheria) when cut or crushed (having resin ducts with terpenes); bark tight, light gray, ± smooth. Stems: cylindric, when young typically < 4 mm diameter, limber, reddish, puberulent on young periderm, knobby at nodes from persistent, short-projecting bases of old petioles (1 mm); containing colorless resin from ducts in stem. Leaves: helically alternate, 3-foliolate, typically 30–50 mm long, petiolate with the 3 leaflets subsessile to sessile arising at same point, without stipules; petiole 5−15 mm long; blades of leaflets ovate to obovate or fan- shaped to rhombic, 5−28 × 5−26 mm, terminal leaflet > lateral leaflets, rounded or obtuse (lateral leaflets) to tapered (terminal leaflets) at base, shallowly to deeply 3-lobed and short-crenate, pinnately veined with principal veins slightly raised on lower surface. Inflorescence: panicle of racemes, on spur shoots clustered at tips of winter stems, panicle to 60 mm long, racemes to 10, 10−15 mm long, each raceme ± 20-flowered, flowers helically arranged and tightly clustered, buds formed in midsummer and flowering starting before leaves, bracteate, densely short-tomentose with brown hairs; peduncle to 5 mm long; bract subtending each branch deltate-broadly awl-shaped and cupped, 1−2 mm long, brownish red, stiff, short-tomentose especially below midpoint, persistent; axes stiff, short-hairy; bractlets subtending pedicel 2, partially hidden by and ⊥ to bract, ovate, 1 mm long, keeled, puberulent at base and on inner surface; pedicel 1−2 mm long increasing in fruit, greenish, sparsely hairy or glabrous. -

Family Scientific Name Life Form Anacardiaceae Spondias Tuberosa
Supplementary Materials: Figure S1 Performance of the gap-filling algorithm on the daily Gcc time-series of the woody cerrado site. The algorithm created, based on an Auto-regressive moving average model (ARMA) fitting over the Gcc time-series, consists of three steps: first, the optimal order of the ARMA model is chosen based on physical principles; secondly, data segments before and after a given gap are fitted using an ARMA model of the order selected in the first step; and next, the gap is interpolated using a weighted function of a forward and a backward prediction based on the models of the selected data segments. The second and third steps are repeated for each gap contained in the entire time series. Table S1 List of plant species identified in the field that appeared in the images retrieved from the digital camera at the caatinga site. Family Scientific name Life form Anacardiaceae Spondias tuberosa Arruda Shrub|Tree Anacardiaceae Myracrodruon urundeuva Allemão Tree Anacardiaceae Schinopsis brasiliensis Engl. Tree Apocynaceae Aspidosperma pyrifolium Mart. & Zucc. Tree Bignoniaceae Handroanthus spongiosus (Rizzini) S.Grose Tree Burseraceae Commiphora leptophloeos (Mart.) J.B.Gillett Shrub|Tree Cactaceae Pilosocereus Byles & Rowley NA Euphorbiaceae Sapium argutum (Müll.Arg.) Huber Shrub|Tree Euphorbiaceae Sapium glandulosum (L.) Morong Shrub|Tree Euphorbiaceae Cnidoscolus quercifolius Pohl Shrub|Tree Euphorbiaceae Manihot pseudoglaziovii Pax & K.Hoffm. NA Euphorbiaceae Croton conduplicatus Kunth Shrub|Sub-Shrub Fabaceae Mimosa tenuiflora (Willd.) Poir. Shrub|Tree|Sub-Shrub Fabaceae Poincianella microphylla (Mart. ex G.Don) L.P.Queiroz Shrub|Tree Fabaceae Senegalia piauhiensis (Benth.) Seigler & Ebinger Shrub|Tree Fabaceae Poincianella pyramidalis (Tul.) L.P.Queiroz NA Malvaceae Pseudobombax simplicifolium A.Robyns Tree Table S2 List of plant species identified in the field that appeared in the images taken at the cerrado shrubland. -

Tree Identification: from Bark and Leaves Or Needles
Tree Identification: from bark and leaves or needles A walk in the woods can be a lot of fun, especially if you bring your kids. How do you get them to come along with you? Tell them this. “Look at the bark on the trees. Can you can find any that look like burnt potato chips, warts, cat scratches, camouflage pants or rippling muscles?” Believe it or not, these are descriptions of different kinds of tree bark. Tree ID from bark:______________________________________________________ Black cherry: The bark looks like burnt potato chips. Hackberry: The bark is bumpy and warty. Ironwood: The bark has long thin strips. With a little imagination, an ironwood can look like it is used as a scratching post by cats. They can also be easily spotted in winter because their light brown dead leaves hang on well past the first snow. Sycamore: A tree with bark that looks like camouflaged pants. The largest tree in Illinois is a sycamore, a majestic 115-foot tree near Springfield. Musclewood: a tree with smooth gray bark covering a trunk with ridges that look like they are rippling muscles. Shagbark hickory: Long strips of shaggy bark peeling at both ends. Cottonwood: Has heavy ridges that make it look like Paul Bunyan’s corduroy pants. Bur oak: Thick and gnarly bark has deep craggy furrows. The corky bark allows it to easily withstand hot forest fires. Tree ID from leaves or needles:________________________________________ Willow Oak: Has elongated leaves similar to those of a willow tree. Red pine: Red has 3 letters; a red pine has groupings of 2-3 prickly needles. -

Schinus Terebinthifolius Anacardiaceae Raddi
Schinus terebinthifolius Raddi Anacardiaceae LOCAL NAMES English (Bahamian holly,Florida holly,christmasberry tree,broadleaf pepper tree,Brazilian pepper tree); French (poivrier du Bresil,faux poivrier); German (Brasilianischer Pfefferbaum); Spanish (pimienta de Brasil,copal) BOTANIC DESCRIPTION S. terebinthifolius is a small tree, 3-10 m tall (ocassionally up to 15 m) and 10-30 cm diameter (occasionally up to 60 cm). S. terebinthifolius may be multi-stemmed with arching, not drooping branches. Tree; taken at: Los Angeles County Arboretum - Arcadia, CA and The National Leaves pinnate, up to 40 cm long, with 2-8 pairs of elliptic to lanceolate Arboretum - Washington, DC (W. Mark and leaflets and an additional leaflet at the end. Leaflets glabrous, 1.5-7.5 cm J. Reimer) long and 7-32 mm wide, the terminal leaflet larger than lateral ones. Leaf margins entire to serrated and glabrous. Flowers white, in large, terminal panicles. Petals oblong to ovate, 1.2-2.5 mm long. Fruits globose, bright red drupes, 4-5 mm in diameter. This is a highly invasive species that has proved to be a serious weed in South Africa, Florida and Hawaii. It is also noted as invasive in other Bark; taken at: Los Angeles County Caribbean and Indian Ocean islands. Rapid growth rate, wide Arboretum - Arcadia, CA and The National environmental tolerance, prolific seed production, a high germination rate, Arboretum - Washington, DC (W. Mark and seedling tolerance of shade, attraction of biotic dispersal agents, possible J. Reimer) allelopathy and the ability to form dense thickets all contribute to this species' success in its exotic range. -
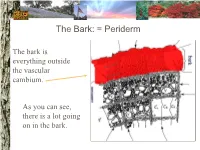
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division. -

Puaiohi Or Small Kaua‘I Thrush (Myadestes Palmeri)
Hawaiian Bird Conservation Action Plan Focal Species: Puaiohi or Small Kaua‘i Thrush (Myadestes palmeri) Synopsis: The Puaiohi is endemic to Kaua’i and is restricted to remote areas of the rugged ‘Alaka’i Plateau. It nests in hollows or on ledges of fern-covered cliffs along narrow streams. The species may always have been rare, and availability of suitable nest sites may limit the range and population size. Habitat management to prevent degradation by non-native plants and feral ungulates is a key to long-term conservation. Artificial nest structures are being investigated as a way of increasing nest site availability and decreasing nest predation. Puaiohi at nest. Photo Eric VanderWerf Typical Puaiohi nesting habitat. Photo KFBRP Geographic region: Kaua‘i, Hawaiian Islands Group: Forest Birds Federal Status: Endangered State status: Endangered IUCN status: Critically Endangered Conservation score, rank: 18/20, At-risk Watch List 2007 Score: RED Juvenile Puaiohi. Photo Eric VanderWerf Climate Change Vulnerability: High Population Size and Trend: The Puaiohi population was estimated to be about 500 birds in 2006 (range 200-1,000; Kaua’i Forest Bird Recovery Project [KFBRP] unpubl. data). The habitat used by Puaiohi is difficult to survey and calculating an accurate population estimate has been challenging. The population trend appears to be stable. In 2011, a new survey method (occupancy modeling; Mackenzie et al. 2006) was field tested and it is hoped that this method will yield a more precise population estimate and provide a more robust method for monitoring trends. Range: The breeding population is restricted to an area of < 20 km2 on the ‘Alaka’i Plateau, and 75% of the population is estimated to occur in just 10 km2 (KFBRP unpubl. -

Intergeneric Graft Compatibility Within the Family Araliaceae
RESEARCH UPDATES Fatshedera ( Fatsia x Hedera) that have Materials and methods Intergeneric been grown erect are sold as novelty specimens. Growers usually get a high Twenty-three cultivars of Graft percentage of successful grafts with Araliaceae representing six genera and Compatibility healthy plant material and good graft- 16 species were obtained from com- ing technique. mercial sources. Two species each of within the Family Variegated forms of Aralia elata two genera native to Hawaii, do not root from cuttings and produce Cheirodendron and Tetraplasandra, Araliaceae nonvariegated seedlings. The varie- were collected in the Koolau Moun- gated forms are propagated by bud- tains on Oahu (Table 1). ding onto seedling or vegetatively Rootstocks propagated from tip Kenneth W. Leonhardt1 produced nonvariegated rootstocks of cuttings rooted in equal parts ver- A. elata (Leiss, 1977). One variegated miculite and perlite under intermit- form of A. elata also has been cleft- tent mist and full sun were grown in Additional index words. Aralia, grafted successfully onto a rootstock 15-cm plastic pots containing equal Ginsing, Panax family, propagation of A. spinosa (Raulston, 1985.) parts peat moss, perlite, and field soil The relative ease of the Hedera x (by volume). Lime and a slow-release Summary. Novelty Araliaceae potted Fatshedera graft raised the possibility granular fertilizer were incorporated. plants were created by a wide variety of graft compatibility of Hedera with Rootstocks were established in a green- of interspecific and intergeneric graft other relatives, particularly those grow- house under 25% shade cover until combinations. Twenty-four species of ing tall rapidly or having other desir- grafted.