University of Birmingham Structural Coloration in Caloenas Nicobarica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
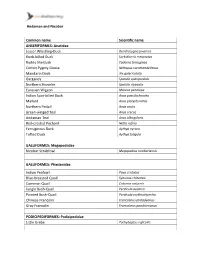
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

Physics of Structural Colors
HOME | SEARCH | PACS & MSC | JOURNALS | ABOUT | CONTACT US Physics of structural colors This article has been downloaded from IOPscience. Please scroll down to see the full text article. 2008 Rep. Prog. Phys. 71 076401 (http://iopscience.iop.org/0034-4885/71/7/076401) The Table of Contents and more related content is available Download details: IP Address: 132.72.138.1 The article was downloaded on 02/07/2008 at 16:04 Please note that terms and conditions apply. IOP PUBLISHING REPORTS ON PROGRESS IN PHYSICS Rep. Prog. Phys. 71 (2008) 076401 (30pp) doi:10.1088/0034-4885/71/7/076401 Physics of structural colors S Kinoshita, S Yoshioka and J Miyazaki Graduate School of Frontier Biosciences, Osaka University, Suita, Osaka 565-0871, Japan E-mail: [email protected] Received 3 September 2007, in final form 16 January 2008 Published 6 June 2008 Online at stacks.iop.org/RoPP/71/076401 Abstract In recent years, structural colors have attracted great attention in a wide variety of research fields. This is because they are originated from complex interaction between light and sophisticated nanostructures generated in the natural world. In addition, their inherent regular structures are one of the most conspicuous examples of non-equilibrium order formation. Structural colors are deeply connected with recent rapidly growing fields of photonics and have been extensively studied to clarify their peculiar optical phenomena. Their mechanisms are, in principle, of a purely physical origin, which differs considerably from the ordinary coloration mechanisms such as in pigments, dyes and metals, where the colors are produced by virtue of the energy consumption of light. -

(2004): Identification, Distribution, and Function of Gastroliths in Dinosaurs
IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Diplodocid sauropod accidentally ingesting gastroliths while feeding on a cycad. IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1. Referent: Privat-Dozent Dr. Martin Sander 2. Referent: Professor Dr. Jes Rust Tag der Promotion: 02.12.2004 Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni- bonn.de/diss_online elektronisch publiziert This dissertation is published electronically on the ULB Bonn server for university publications: http://hss.ulb.uni-bonn.de/diss_online Dedicated to Claudia. Thanks for everything. French proverb: “Il a un estomac d’autuche!” (literally: He has the stomach of an ostrich!) means: He can tolerate everything! Geheimnisvoll am lichten Tag Läßt sich Natur des Schleiers nicht berauben, Und was sie deinem Geist -

A Synopsis of the Pre-Human Avifauna of the Mascarene Islands
– 195 – Paleornithological Research 2013 Proceed. 8th Inter nat. Meeting Society of Avian Paleontology and Evolution Ursula B. Göhlich & Andreas Kroh (Eds) A synopsis of the pre-human avifauna of the Mascarene Islands JULIAN P. HUME Bird Group, Department of Life Sciences, The Natural History Museum, Tring, UK Abstract — The isolated Mascarene Islands of Mauritius, Réunion and Rodrigues are situated in the south- western Indian Ocean. All are volcanic in origin and have never been connected to each other or any other land mass. Despite their comparatively close proximity to each other, each island differs topographically and the islands have generally distinct avifaunas. The Mascarenes remained pristine until recently, resulting in some documentation of their ecology being made before they rapidly suffered severe degradation by humans. The first major fossil discoveries were made in 1865 on Mauritius and on Rodrigues and in the late 20th century on Réunion. However, for both Mauritius and Rodrigues, the documented fossil record initially was biased toward larger, non-passerine bird species, especially the dodo Raphus cucullatus and solitaire Pezophaps solitaria. This paper provides a synopsis of the fossil Mascarene avifauna, which demonstrates that it was more diverse than previously realised. Therefore, as the islands have suffered severe anthropogenic changes and the fossil record is far from complete, any conclusions based on present avian biogeography must be viewed with caution. Key words: Mauritius, Réunion, Rodrigues, ecological history, biogeography, extinction Introduction ily described or illustrated in ships’ logs and journals, which became the source material for The Mascarene Islands of Mauritius, Réunion popular articles and books and, along with col- and Rodrigues are situated in the south-western lected specimens, enabled monographs such as Indian Ocean (Fig. -

Pollia Condensata Has an Extraordinary Blue Fruit Known for Its High Intensity Reflectivity, and Glittery Appearance
LIVING LIGHT 2018 3 CONTENTS Introduction ....................................................... 3 Scientific commitee ............................................. 4 Local organising commitee ................................... 4 Graphics ............................................................ 4 History .............................................................. 5 Timetable .......................................................... 6 Find us .............................................................11 Abstracts, invited lectures ...................................13 Abstracts, lectures .............................................21 Abstracts, posters ..............................................67 List of participants .............................................92 Contacts...........................................................96 Sponsors ..........................................................97 Notes ...............................................................98 4 5 INTRODUCTION Dear Living Light 2018 Participant, It is an honour for us to host such an exciting meeting in Cambridge and we thank you for joining us! We designed the meeting with the intention of maximising interaction between the participants and with the hope that you will go back home with a new set of collaborators and friends which are as passionate as you are about working at the interface between biology, chemistry, physics, and engineering! We truly hope that you will enjoy the conference and we ask for your collaboration to keep the meeting -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Notices
21262 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Notices acquisition were not included in the 5275 Leesburg Pike, Falls Church, VA Comment (1): We received one calculation for TDC, the TDC limit would not 22041–3803; (703) 358–2376. comment from the Western Energy have exceeded amongst other items. SUPPLEMENTARY INFORMATION: Alliance, which requested that we Contact: Robert E. Mulderig, Deputy include European starling (Sturnus Assistant Secretary, Office of Public Housing What is the purpose of this notice? vulgaris) and house sparrow (Passer Investments, Office of Public and Indian Housing, Department of Housing and Urban The purpose of this notice is to domesticus) on the list of bird species Development, 451 Seventh Street SW, Room provide the public an updated list of not protected by the MBTA. 4130, Washington, DC 20410, telephone (202) ‘‘all nonnative, human-introduced bird Response: The draft list of nonnative, 402–4780. species to which the Migratory Bird human-introduced species was [FR Doc. 2020–08052 Filed 4–15–20; 8:45 am]‘ Treaty Act (16 U.S.C. 703 et seq.) does restricted to species belonging to biological families of migratory birds BILLING CODE 4210–67–P not apply,’’ as described in the MBTRA of 2004 (Division E, Title I, Sec. 143 of covered under any of the migratory bird the Consolidated Appropriations Act, treaties with Great Britain (for Canada), Mexico, Russia, or Japan. We excluded DEPARTMENT OF THE INTERIOR 2005; Pub. L. 108–447). The MBTRA states that ‘‘[a]s necessary, the Secretary species not occurring in biological Fish and Wildlife Service may update and publish the list of families included in the treaties from species exempted from protection of the the draft list. -

Nicobar Pigeon Caloenas Nicobarica
Nicobar Pigeon Caloenas nicobarica Class: Aves Order: Columbiformes Family: Columbidae Characteristics: Also known as the hackled pigeon, vulturine pigeon and white-tailed pigeon, the nicobar pigeon is medium-sized pigeon with a grey chest and head, metallic back and wings and a white tail. Its long feathers trailing down from the neck give it its distinct look (Lincoln Park Zoo). Behavior: Nicobar pigeons are nomadic, commuting between islands around New Guinea is flocks of up to 85 birds. They will only roost and breed on islands with no humans (Who Zoo). Reproduction: Nicobar pigeon males may spend days courting a female but, if she accepts him in the end, it pays off as they mate for life. The male chooses the nest site and brings twigs and other plant material to the female who builds the Range & Habitat: nest. One egg is produced per clutch and they usually clutch twice per Forests on uninhabited islands year. Both parents incubate the egg which hatches after about 30 days. The chicks stays in the nest for about a month (Rosamond Gifford Zoo). Diet: Wild: Hard seeds, fruit, insects, corn Zoo: Fruits, vegetables, greens, pheasant grains Conservation: Lifespan: up to 15 years in Nicobar pigeon numbers are declining. They fall victim to the pet trade, captivity, 8-12 years in the wild. logging on islands, and are trapped for food. Special Adaptations: Have a very FYI: muscular gizzard that allows them Unique to pigeons, they drink by sticking their beak in the water but don’t to eat nuts with very hard shells. -

West New Britain Extension July 22–27, 2017
WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 White-mantled Kingfisher (Dion Hobcroft) LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 By Dion Hobcroft A Purple-bellied Lory dines out in a Coconut flower. (Dion Hobcroft) Our tour got off to a shaky start with a cancelled flight from Port Moresby to Hoskins. Luckily, the superb Airways Hotel honored our rooms from the previous day that we had missed due to another cancelled flight. Love you Air Niugini! After settling in to the very comfortable Walindi Dive Resort, with its well-planned rooms, tasty meals, and excellent staff, we headed out for our first birding in New Britain. We met Joel, a local villager, who guided us up beyond his village into the forest. He led us to a New Britain Boobook, a small hawk-owl he keeps tabs on (and has done so for the past few years). With the elections on, I joked with Joel that we should call the owl “Prime Minister Pete.” We had actually seen the Prime Minister the day before. Victor Emanuel Nature Tours 2 West New Britain Extension, 2017 After great looks at “Pete” we slowly wandered to a viewing area over the forest that was heaving with birds —lots of Eclectus Parrots, our first Blue-eyed Cockatoos, dozens of Red-knobbed and Yellowish imperial-pigeons, Long-tailed Myna, Variable Goshawk, an all-white Pied Coucal, a single Channel-billed Cuckoo (quite rare on NB), the beautiful Purple-bellied Lory, the scarce Black-bellied Myzomela, and great views of the colorful Knob-billed Fruit-Dove. -

Palau Bird Survey Report 2020
Abundance of Birds in Palau based on Surveys in 2005 Final Report, November 2020 Eric A. VanderWerf1 and Erika Dittmar1 1 Pacific Rim Conservation, 3038 Oahu Avenue, Honolulu, Hawaii 96822 Prepared for the Belau National Museum, Box 666, Koror Palau 96940 Endemic birds of Palau, from top left: White-breasted Woodswallow, Palau Fantail, Palau Fruit- dove, Rusty-capped Kingfisher. Photos by Eric VanderWerf. 1 TABLE OF CONTENTS ACKNOWLEDGMENTS .............................................................................................................. 3 EXECUTIVE SUMMARY ............................................................................................................ 4 INTRODUCTION .......................................................................................................................... 5 METHODS ..................................................................................................................................... 6 Description of Study Area and Transect Locations ............................................................ 6 Data Collection ................................................................................................................... 7 Data Analysis ...................................................................................................................... 7 Limitations of the Survey.................................................................................................... 9 RESULTS .................................................................................................................................... -

Kea (Nestor Notabilis) Care Manual
Kea (Nestor notabilis) CARE MANUAL CREATED BY THE AZA Kea Species Survival Plan® Program IN ASSOCIATION WITH THE AZA Parrot Taxon Advisory Group Kea (Nestor notabilis) Care Manual Kea (Nestor notabilis) Care Manual Published by the Association of Zoos and Aquariums in collaboration with the AZA Animal Welfare Committee Formal Citation: AZA Kea Species Survival Plan (Nestor notabilis). (2020). Kea Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: July 1, 2019 Kea (Nestor notabilis) Care Manual Coordinator: Kimberly Klosterman, Cincinnati Zoo & Botanical Garden, Senior Avian Keeper, Kea SSP Vice Coordinator Authors and Significant Contributors: Krista Adlehart CRM, Woodland Park Zoo, Animal Management Registrar Amanda Ardente NVM, PhD, Walt Disney World, University of Florida, Nutrition Fellow Jackie Bray, MA Zoology CPBT-KA, Raptor Incorporated, Associate Director Cassandre Crawford MM, Northwest Local School District, Orchestra Director, Kea SSP Volunteer Thea Etchells, Denver Zoo, Bird Keeper Linda Henry, Board Member of Zoological Lighting Institute, SeaWorld San Diego Phillip Horvey, Sedgwick County Zoo, Senior Zookeeper, Masked Lapwing SSP Coordinator and Studbook Keeper Cari Inserra, San Diego Zoo, Lead Animal Trainer Kimberly Klosterman, Cincinnati Zoo & Botanical Garden, Senior Avian Keeper, Kea Care Manual Coordinator, Vice Coordinator Kea SSP Program Jessica Meehan, Denver Zoo, Bird Keeper, Kea SSP Coordinator and Studbook Keeper Jennifer Nollman DVM, Cincinnati Zoo & Botanical Garden, Associate Veterinarian Catherine Vine, Philadelphia Zoo, Avian Keeper Reviewers: Raoul Schwing PhD, Head of Kea Lab & Infrastructure Project Manager, Messerli Research Institute, University of Vienna, AU Tamsin Orr-Walker, BAAT, Co-founder, Trustee & Chair of Kea Conservation Trust, South Island Community Engagement Coordinator, NZ Nigel Simpson, EAZA Kea EEP Coordinator, Head of Operations, Wild Place Project, Bristol Zoological Society, UK Dr.rer.nat Gyula K. -

The Optical Janus Effect: Asymmetric Structural Color Reflection Materials
The Optical Janus Effect: Asymmetric Structural Color Reflection Materials The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation England, Grant T., Calvin Russell, Elijah Shirman, Theresa Kay, Nicolas Vogel, and Joanna Aizenberg. 2017. “The Optical Janus Effect: Asymmetric Structural Color Reflection Materials.” Advanced Materials 29 (29) (June 6): 1606876. doi:10.1002/adma.201606876. Published Version doi:10.1002/adma.201606876 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:37235483 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP DOI: 10.1002/ ((please add manuscript number)) Article type: Full Paper The Optical Janus Effect: Asymmetric Structural Color Reflection Materials Grant T. Englanda, Calvin Russella, Elijah Shirmanab, Theresa Kayb, Nicolas Vogelc, and Joanna Aizenberg*abd aJohn A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, USA bWyss Institute for Biologically Inspired Engineering, Harvard University, Cambridge, USA cInstitute of Particle Technology, Friedrich-Alexander University Erlangen-Nürnberg, Cauerstrasse 4, 91058 Erlangen, Germany dDepartment of Chemistry and Chemical Biology, Harvard University, Cambridge, USA Keywords: structural color, absorption, multilayer, photonic crystals, thin film. Abstract: Structurally colored materials are often used for their resistance to photobleaching and their complex viewing direction-dependent optical properties. Frequently, absorption has been added to these types of materials in order to improve the color saturation by mitigating the effects of nonspecific scattering that is present in most samples due to imperfect manufacturing procedures. -

Of the Mascarene Islands, with Three New Species
Zootaxa 3124: 1–62 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 3124 Systematics, morphology, and ecology of pigeons and doves (Aves: Columbidae) of the Mascarene Islands, with three new species JULIAN PENDER HUME Correspondence Address: Bird Group, The Department of Zoology, Natural History Museum, Akeman St, Tring, Herts HP23 6AP. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by S. Olson: 11 Oct. 2011; published: 08 Dec. 2011 JULIAN PENDER HUME Systematics, morphology, and ecology of pigeons and doves (Aves: Columbidae) of the Mascarene Islands, with three new species (Zootaxa 3124) 62 pp.; 30 cm. 08 Dec. 2011 ISBN 978-1-86977-825-5 (paperback) ISBN 978-1-86977-826-2 (Online edition) FIRST PUBLISHED IN 2011 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2011 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 3124 © 2011 Magnolia Press HUME Table of contents Abstract . 3 Introduction . 3 Materials and methods . 5 Systematics . 6 Discussion .