Cyproconazole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

US 2014/0116112 A1 HUMPHREY Et Al
US 201401 16112A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0116112 A1 HUMPHREY et al. (43) Pub. Date: May 1, 2014 (54) METHODS FOR DETERMINING THE Publication Classification PRESENCE OR ABSENCE OF CONTAMINANTS IN A SAMPLE (51) Int. Cl. GOIN30/72 (2006.01) (71) Applicant: K & D LABORATORIES, INC., Lake (52) U.S. Cl. Oswego, OR (US) CPC .................................. G0IN30/7206 (2013.01) USPC ......................................................... T3/23.37 (72) Inventors: David Kent HUMPHREY, Reno, NV (US); Nicholas Joseph GEISE, Portland, OR (US) (57) ABSTRACT (73) Assignee: K & D LABORATORIES, INC., Lake Oswego, OR (US) Methods are provided for rapidly determining the presence or absence of large numbers of contaminants in a test sample, (21) Appl. No.: 13/830,388 Such as a raw material intended for use in the preparation of a nutraceutical. The disclosed methods employ gas chromatog (22) Filed: Mar 14, 2013 raphy-mass spectrometry techniques together with the spe cific use of software in combination with a database to ana Related U.S. Application Data lyze data collected after ionization of the sample and (60) Provisional application No. 61/718,607, filed on Oct. determine the presence or absence of the contaminants in the 25, 2012. sample. US 2014/01161 12 A1 May 1, 2014 METHODS FOR DETERMINING THE 0007. In one embodiment, methods for detecting the pres PRESENCE OR ABSENCE OF ence or absence of a plurality of contaminants in a sample are CONTAMINANTS IN A SAMPLE provided, such methods comprising: (a) extracting the sample with a water-miscible solvent in the presence of a high con REFERENCE TO RELATED APPLICATIONS centration of salts to provide a sample extract; (b) shaking and centrifuging the sample extract to provide a Supernatant; (c) 0001. -
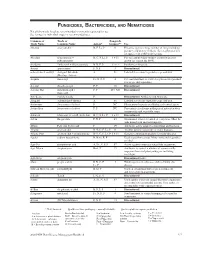
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Multi-Omics Phenotyping of the Gut-Liver Axis Reveals Metabolic Perturbations from a Low-Dose Pesticide Mixture in Rats
ARTICLE https://doi.org/10.1038/s42003-021-01990-w OPEN Multi-omics phenotyping of the gut-liver axis reveals metabolic perturbations from a low-dose pesticide mixture in rats Robin Mesnage 1, Maxime Teixeira 2, Daniele Mandrioli 3, Laura Falcioni 3, Mariam Ibragim1, Quinten Raymond Ducarmon4, Romy Daniëlle Zwittink 4, Caroline Amiel2, Jean-Michel Panoff2, ✉ Emma Bourne5, Emanuel Savage5, Charles A. Mein5, Fiorella Belpoggi3 & Michael N. Antoniou 1 Health effects of pesticides are not always accurately detected using the current battery of regulatory toxicity tests. We compared standard histopathology and serum biochemistry 1234567890():,; measures and multi-omics analyses in a subchronic toxicity test of a mixture of six pesticides frequently detected in foodstuffs (azoxystrobin, boscalid, chlorpyrifos, glyphosate, imida- cloprid and thiabendazole) in Sprague-Dawley rats. Analysis of water and feed consumption, body weight, histopathology and serum biochemistry showed little effect. Contrastingly, serum and caecum metabolomics revealed that nicotinamide and tryptophan metabolism were affected, which suggested activation of an oxidative stress response. This was not reflected by gut microbial community composition changes evaluated by shotgun metage- nomics. Transcriptomics of the liver showed that 257 genes had their expression changed. Gene functions affected included the regulation of response to steroid hormones and the activation of stress response pathways. Genome-wide DNA methylation analysis of the same liver samples showed that 4,255 CpG sites were differentially methylated. Overall, we demonstrated that in-depth molecular profiling in laboratory animals exposed to low con- centrations of pesticides allows the detection of metabolic perturbations that would remain undetected by standard regulatory biochemical measures and which could thus improve the predictability of health risks from exposure to chemical pollutants. -

Pest Management Strategic Plan for Western U.S. Sugarbeet Production
Pest Management Strategic Plan for Western U.S. Sugarbeet Production Summary of a workshop held on December 15–16, 2004 Boise, ID Issued 08/05/05 Lead Authors: Ronda Hirnyck, Lisa Downey Editor: Sally O’Neal Coates Contact Person: Ronda Hirnyck, Idaho Pesticide Program Coordinator (208) 364-4046 [email protected] This project was sponsored by the Western Integrated Pest Management Center, which is funded by the United States Department of Agriculture, Cooperative State Research, Education and Extension Service. Table of Contents Work Group................................................................................................3 Summary of Critical Needs .......................................................................5 Introduction ................................................................................................7 Production Overview ..................................................................................9 Foundation for the Pest Management Strategic Plan Crop Rotation ...................................................................................13 Pre-plant ...........................................................................................14 Planting.............................................................................................24 Pre-emergence ..................................................................................39 Emergence to Row Closure...............................................................44 Row Closure to Harvest....................................................................71 -

U.S. EPA, Pesticide Product Label, QUADRIS XTRA FUNGICIDE, 05/06/2008
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES Ms. Adora Clark MAY 6 2008 Syngenta Crop Protection, Inc. ~ P.O. Box 18300 Greensboro, NC 27419 Dear Ms Clark: Subject: Quadris Xtra Fungicide EPA Reg. No. 100-1225 Your Revised Label of February 8, 2008 The amendment referred to above, submitted in connection with registration under FIFRA sec. 3(c)(7)(A), is conditionally accepted provided that the following revision is made to the label: Update the Warranty Statement tocohform to that for Tilt, EPA Reg. No. 100-617, accepted May 25,2007. Submit one copy of the final printed label before you release the product for shipment. If these conditions are not complied with, the registration will be subject to cancellation in accordance with FIFRA sec. 6(e). Your release for shipment of the product constitutes acceptance of these conditions. A stamped copy of the label is enclosed for your records. Sincerely yours, C o-J5'~~~1t'- Mary L Waller Product Manager 21 Fungicide Branch Registration Division (750 Enclosures 7505P:CGrable:cg:5/1/08 ~, ' "'," ,t U-r'.,' Page 18 'CONTAINER/BASE LABEL I GROUP _ FUNGICIDES I Quadris Xtra ™ Fungicide Broad spectrum fungicide for control of plant diseases Active Ingredients: "Azoxy~trobin(CAS No. 131-860-33:'8} ....................... , .............................. 18.2% 'Cypr6c6naiole (cAs No. 94361-'06;;,5).. ............. , .. : .................................... 7.3% Other Ingredients: 74.5% Total: 100.0% Quadris Xtra is a 280SC formulation that contains 0.67 lb. a.i. cyproconazole and 1.67 lb. a.i. azoxystrobin per gallon. ~.!.:~(:~~r.T~D . -

US EPA, Pesticide Product Label, Azoxystrobin 2SC,08/06/2020
U.S. ENVIRONMENTAL PROTECTION AGENCY EPA Reg. Number: Date of Issuance: Office of Pesticide Programs Registration Division (7505P) 94730-6 8/6/20 1200 Pennsylvania Ave., N.W. Washington, D.C. 20460 NOTICE OF PESTICIDE: Term of Issuance: X Registration Reregistration Unconditional (under FIFRA, as amended) Name of Pesticide Product: Azoxystrobin 2SC Name and Address of Registrant (include ZIP Code): Generic Crop Science, LLC c/o Biologic Regulatory Consulting, Inc. 10529 Heritage Bay Blvd. Naples, FL 34120 Note: Changes in labeling differing in substance from that accepted in connection with this registration must be submitted to and accepted by the Registration Division prior to use of the label in commerce. In any correspondence on this product always refer to the above EPA registration number. On the basis of information furnished by the registrant, the above named pesticide is hereby registered under the Federal Insecticide, Fungicide and Rodenticide Act. Registration is in no way to be construed as an endorsement or recommendation of this product by the Agency. In order to protect health and the environment, the Administrator, on his motion, may at any time suspend or cancel the registration of a pesticide in accordance with the Act. The acceptance of any name in connection with the registration of a product under this Act is not to be construed as giving the registrant a right to exclusive use of the name or to its use if it has been covered by others. This product is unconditionally registered in accordance with FIFRA section 3(c)(5) provided that you: 1. Submit and/or cite all data required for registration/reregistration/registration review of your product when the Agency requires all registrants of similar products to submit such data. -

Strobilurins: Evolution of a New Class of Active Substances
Strobilurins––new fungicides for crop protection Kresoxim-methyl REVIEWS Strobilurins: Evolution of a New Class of Active Substances Hubert Sauter,* Wolfgang Steglich, and Timm Anke Dedicated to Professor Hans-Jürgen Quadbeck-Seeger on the occasion of his 60th birthday Nature provides active substances, as mansins, the identification of the b- ture ± activity relationships. The varia- the result of special biosynthetic path- methoxyacrylate group as their com- tion of natural product lead structures ways, with the most diverse biological mon structural element, and the eluci- and the selection of suitable candidates activities. Many of these substances dation of their mode of actionÐsaw for development led to an across-the- perform functions that are important the focus of research activities shift board evolution of the strobilurins as a to the survival of the organism in which away from the universities, ushering in new class of active substance. Several they are formed. For example, certain an era of intense competition between commercial products resulting from fungi produce antifungal strobilurins rival industrial fungicide research this effort are now already on the and oudemansins, agents to which the teams. Their goal was, and remains, market, and more are expected to producers themselves are not sensitive, the development of fungicides with follow: a most intriguing success story. but which ward off other fungal species practical applications in agricultural that grow on the same substrates. The crop protection. This review illustrates Keywords: active substance research period that followed the early break- methodological approaches in the op- ´ fungicides ´ natural products ´ throughs in the 1970sÐthe discovery timization of lead structures and the strobilurins ´ structure ± activity re- of the natural strobilurins and oude- importance of understanding struc- lationships 1. -

Interactive Effect of Nitrogen and Azoxystrobin on Yield, Quality, Nitrogen and Water Use Efficiency of Wild Rocket in Southern
agronomy Article Interactive Effect of Nitrogen and Azoxystrobin on Yield, Quality, Nitrogen and Water Use Efficiency of Wild Rocket in Southern Italy Vincenzo Candido 1,*, Francesca Boari 2, Vito Cantore 2 , Donato Castronuovo 1 , Donato Di Venere 2, Michele Perniola 3, Lucrezia Sergio 2, Roberto Viggiani 1 and Maria Immacolata Schiattone 1,* 1 School of Agriculture, Forest, Food and Environmental Sciences, University of Basilicata, Viale dell’Ateneo Lucano, 10, 85100 Potenza, Italy; [email protected] (D.C.); [email protected] (R.V.) 2 Institute of Sciences of Food Production, National Research Council (CNR-ISPA), Via Amendola, 122/O, 70125 Bari, Italy; [email protected] (F.B.); [email protected] (V.C.); [email protected] (D.D.V.); [email protected] (L.S.) 3 Department of European and Mediterranean Cultures, University of Basilicata, Via Lanera, 10, 75100 Matera, Italy; [email protected] * Correspondence: [email protected] (V.C.); [email protected] (M.I.S.) Received: 14 May 2020; Accepted: 12 June 2020; Published: 14 June 2020 Abstract: Wild rocket (Diplotaxis tenuifolia L. DC) is an emerging vegetable which market requires high-quality standards that can be obtained through appropriate cultivation techniques such as the right level of nitrogen and the application of biostimulant substances. These include strobilurins, marketed mainly as fungicides that can have complementary positive effects on the yield, quality and resources’ use efficiency of many crops. For this reason, a trial in an unheated greenhouse, in Southern Italy, to evaluate the possibility of using Azoxystrobin to improve the production of wild 1 rocket subjected to different nitrogen inputs, was carried out. -

A Critical Review of Synthetic Chemicals in Surface Waters of the US, the EU and China
Environment International 131 (2019) 104994 Contents lists available at ScienceDirect Environment International journal homepage: www.elsevier.com/locate/envint Review article A critical review of synthetic chemicals in surface waters of the US, the EU T and China ⁎ Wendi Fanga, Ying Penga, Derek Muirb,c, Jun Lind, Xiaowei Zhanga, a State Key Laboratory of Pollution Control & Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, PR China b Environment and Climate Change Canada, Canada Centre for Inland Waters, 867 Lakeshore Road, Burlington, ON L7S 1A1, Canada c School of Environment, Jinan University, Guangzhou, PR China d Solid Waste and Chemicals Management Center, Ministry of Ecology and Environment, PR China ARTICLE INFO ABSTRACT Handling editor: Adrian Covaci There is a wide concern that emerging organic pollutants (EOPs) in surface water could adversely affect human Keywords: health and wildlife. However, the geographic distribution, exposure pattern and ecological risk of emerging Chemical pollution organic pollutants are poorly understood at a global scale. This paper provides a comprehensive survey on the Risk assessment exposure level of EOPs in China, the US and the EU based on the published literature. The hazard level of three Pesticide categories of EOPs, namely pharmaceuticals and personal care products (PPCPs), pesticides and industrial Industry chemicals was further evaluated by adopting a novel Aquatic HazPi index that jointly accounts for the persis- Pharmaceutical and personal care products tence, bioaccumulation, toxicity and bioactivity. Furthermore, a correlation analysis of land use with the surface Land use water exposure status regarding the synthetic chemicals was conducted. According to the published data re- ported between 2010 and 2016, the concentration of pesticides in the US was higher than in the EU and China. -

Natural Compound-Derived Cytochrome Bc1 Complex Inhibitors As Antifungal Agents
molecules Review Natural Compound-Derived Cytochrome bc1 Complex Inhibitors as Antifungal Agents Loana Musso * , Andrea Fabbrini and Sabrina Dallavalle Department of Food, Environmental and Nutritional Sciences (DeFENS), Division of Chemical and Biomolecular Sciences, Università degli Studi di Milano, Via Celoria 2, 20133 Milan, Italy; [email protected] (A.F.); [email protected] (S.D.) * Correspondence: [email protected] Received: 4 August 2020; Accepted: 6 October 2020; Published: 7 October 2020 Abstract: The high incidence of fungal pathogens has become a global issue for crop protection. A promising strategy to control fungal plant infections is based on the use of nature-inspired compounds. The cytochrome bc1 complex is an essential component of the cellular respiratory chain and is one of the most important fungicidal targets. Natural products have played a crucial role in the discovery of cytochrome bc1 inhibitors, as proven by the development of strobilurins, one of the most important classes of crop-protection agents, over the past two decades. In this review, we summarize advances in the exploration of natural product scaffolds for the design and development of new bc1 complex inhibitors. Particular emphasis is given to molecular modeling-based approaches and structure–activity relationship (SAR) studies performed to improve the stability and increase the potency of natural precursors. The collected results highlight the versatility of natural compounds and provide an insight into the potential development of nature-inspired derivatives as antifungal agents. Keywords: natural compounds; cytochrome bc1 complex; antifungals; (E)-β-methoxyacrylate; strobilurins 1. Introduction Plant diseases caused by fungal pathogens are a major threat to the agricultural industry worldwide. -

Recognition and Management of Pesticide Poisonings: Fungicides
CHAPTER 16 HIGHLIGHTS Numerous fungicides in use with varying levels of toxicity Fungicides Most are unlikely to cause systemic poisonings, exceptions being Fungicides are extensively used in industry, agriculture and the home and garden for: • Organomercury 1. protection of seed grain during storage, shipment and germination; compounds • Triazoles 2. protection of mature crops, berries, seedlings, flowers and grasses in the field, in storage and during shipment; • Some copper compounds 3. suppression of mildews that attack painted surfaces; • Isolated EBDC 4. control of slime in paper pulps; and exceptions 5. protection of carpet and fabrics in the home. Approximately 500 million pounds of fungicides are applied worldwide annu- ally (see Chapter 1, Introduction). Fungicides vary enormously in their potential for causing adverse effects in humans. Historically, some of the most tragic epidemics of pesticide poisoning occurred by mistaken consumption of seed grain treated with organic mercury or hexachlorobenzene. However, most fungicides currently in use and registered for use in the United States are unlikely to cause frequent or severe acute systemic poison- ings for several reasons: (1) many have low inherent toxicity in mammals and are inefficiently absorbed; (2) many are formulated as suspensions of wettable powders or granules, from which rapid, efficient absorption is unlikely; and (3) methods of application are such that relatively few individuals are intensively exposed. Apart from systemic poisonings, fungicides as a class also cause irritant injuries to skin and mucous membranes, as well as some dermal sensitization. The following discussion considers the recognized adverse effects of widely used fungicides. In the case of those agents that have caused systemic poisoning, some recommendations for management of poisonings and injuries are provided. -
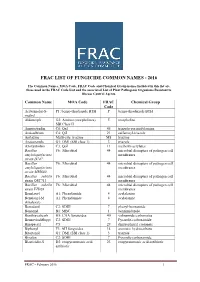
Frac List of Fungicide Common Names - 2016
FRAC LIST OF FUNGICIDE COMMON NAMES - 2016 The Common Names, MOA Code, FRAC Code and Chemical Group names included in this list are those used in the FRAC Code List and the associated List of Plant Pathogenic Organisms Resistant to Disease Control Agents. Common Name MOA Code FRAC Chemical Group Code Acibenzolar-S- P1: benzo-thiadiazole BTH P benzo-thiadiazole BTH methyl Aldimorph G2: Amines (morpholines) 5 morpholine SBI Class II Ametoctradin C8: QxI 45 triazolo-pyrimidylamine Amisulbrom C4: QiI 21 sulfamoyl-triazole Anilazine Multi-site: triazine M8 triazine Azaconazole G1: DMI (SBI class 1) 3 triazole Azoxystrobin C3: QoI 11 methoxy-acrylates Bacillus F6: Microbial 44 microbial disrupters of pathogen cell amyloliquefaciens membranes strain D747 Bacillus F6: Microbial 44 microbial disrupters of pathogen cell amyloliquefaciens membranes strain MBI600 Bacillus subtilis F6: Microbial 44 microbial disrupters of pathogen cell strain QST713 membranes Bacillus subtilis F6: Microbial 44 microbial disrupters of pathogen cell strain FZB24 membranes Benalaxyl A1: Phenylamide 4 acylalanine Benalaxyl-M A1: Phenylamide 4 acylalanine (kiralaxyl) Benodanil C2: SDHI 7 phenyl-benzamide Benomyl B1: MBC 1 benzimidazole Benthiavalicarb H5: CAA fungicides 40 valinamide carbamates Benzovindiflupyr C2: SDHI 7 Pyrazole-carboxamide Binapacryl C5 29 dinitrophenyl crotonate Biphenyl F3: AH fungicides 14 aromatic hydrocarbons Bitertanol G1: DMI (SBI class 1) 3 triazole Bixafen C2: SDHI 7 Pyrazole-carboxamide Blasticidin-S D2: enopyranuronic acid 23 enopyranuronic