Abc Transporters of the Wheat Pathogen Mycosphaerella Graminicola
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
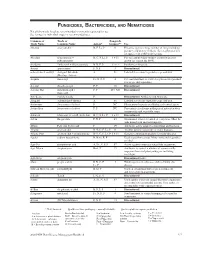
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Topical and Systemic Antifungal Therapy for Chronic Rhinosinusitis (Protocol)
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of East Anglia digital repository Cochrane Database of Systematic Reviews Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Head K, Sacks PL, Chong LY, Hopkins C, Philpott C Head K, Sacks PL, Chong LY, Hopkins C, Philpott C. Topical and systemic antifungal therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No.: CD012453. DOI: 10.1002/14651858.CD012453. www.cochranelibrary.com Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 BACKGROUND .................................... 1 OBJECTIVES ..................................... 3 METHODS ...................................... 3 ACKNOWLEDGEMENTS . 8 REFERENCES ..................................... 9 APPENDICES ..................................... 10 CONTRIBUTIONSOFAUTHORS . 25 DECLARATIONSOFINTEREST . 26 SOURCESOFSUPPORT . 26 NOTES........................................ 26 Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) i Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. [Intervention Protocol] Topical and systemic antifungal therapy for chronic rhinosinusitis Karen Head1, Peta-Lee Sacks2, Lee Yee Chong1, Claire Hopkins3, Carl Philpott4 1UK Cochrane Centre, -

Certain Pharmaceuticals and Intermediate Chemicals: Identification of Applicable 6-Digit HS Subheadings for Products Covered By
Second Interim Report to the Public on CERTAIN PHARMACEUTICALS AND INTERMEDIATE CHEMICALS: IDENTIFI CATION OF APPLICABLE 6-DIGIT HS SUBHEADINGS. .· FOR PRODUCTS ·coVERED BY THE PROPOSED URUGUAY ROUND PHARMACEUTICAL A,GREEMENT Investigation No. 332-322 USITC PUBLICATION 2500 APRIL 1992 ,f~· United States International Trade Commission . ·J, Washington, DC 20436 . ·-~}~~:; . _:, -~;<$f ·!•;. UNITED STATES INTERNATIONAL TRADE COMMISSION COMMISSIONERS Don E. Newquist, Chairman Anne E. Brunsdale, Vice Chairman David B. Rohr Carol T. Crawford Janet A. Nuzum Peter S. Watson Office of Operations Charles W. Ervin, Director Office of Industries Rohen A. Rogowsky, Director This report was prepared principally by Eli?.abcth R. Ncsbiu Project Leader Aimison Jonnard Edward Matusik David Michels James Raftery Office of Industries David Beck Office of Tariff Affairs and Trade Agreements With assistance from Paul Daniels James Gill Office ofInformation Resources Management Under the direction of John J. Gersic, Chief Energy & Chemicals Division and Edmund D. Cappuccilli, Chief Energy, Petroleum, Benzenoid Chemicals, and Rubber and Plastics Branch · Energy & Chemicals Division Additional assistance provided by Brenda Carroll and Keilh Hipp With special assistance from U.S. Customs Service Address all communications to Kenneth R. Mason, Secreta·ry to the Commission United States International Trade Commission Washington, DC 20436 PREFACE This report is the second of two interim reports to the public pertaining to Commission investigation No. 332-322, entitled Certain Pharmaceuticals and Intermediate Chemicals; Identification of Applicable 6-Digit HS Subheadings for Products Covered by the Proposed Uruguay Round Pharmaceutical Agreement. The investigation was instituted following receipt of a letter from the United States Trade Representative (USTR) on January 27, 1992, requesting that the Commission conduct an investigation under section 332(g) of the Tariff Act of 1930 (see appendix A for a copy of the USTR' s request)·. -

Discovery of New Antimicrobial Agents Using Combinatorial Chemistry
Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2007 Discovery of New Antimicrobial Agents using Combinatorial Chemistry William I. Northern Wright State University Follow this and additional works at: https://corescholar.libraries.wright.edu/etd_all Part of the Immunology and Infectious Disease Commons, and the Microbiology Commons Repository Citation Northern, William I., "Discovery of New Antimicrobial Agents using Combinatorial Chemistry" (2007). Browse all Theses and Dissertations. 206. https://corescholar.libraries.wright.edu/etd_all/206 This Thesis is brought to you for free and open access by the Theses and Dissertations at CORE Scholar. It has been accepted for inclusion in Browse all Theses and Dissertations by an authorized administrator of CORE Scholar. For more information, please contact [email protected]. DISCOVERY OF NEW ANTIMICROBIAL AGENTS USING COMBINATORIAL CHEMISTRY A thesis submitted in partial fulfillment Of the requirements for the degree of Master of Science By WILLIAM ISAAC NORTHERN B.S., University of Cincinnati, 1982 2007 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES October 29, 2007 I HEREBY RECOMMEND THAT THE THESIS PREPAIRED UNDER MY SUPERVISION BY William Isaac Northern ENTITLED Discovery of New Antimicrobial Agents Using Combinatorial Chemistry BE ACCEPTED IN PARTIAL FULFULLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science ______________________________ Nancy Bigley, Ph.D. Thesis Director ______________________________ Barbara Hull, Ph.D. Department Chair Committee on Final Examination ______________________________ Nancy Bigley. Ph.D. ______________________________ Barbara Hull, Ph.D. ______________________________ Daniel Ketcha, Ph.D. ______________________________ Gerald Alter, Ph.D. ______________________________ Joseph F. Thomas, Jr., Ph.D. Dean, School of Graduate Studies ABSTRACT Northern, William Isaac. -

Fungi P1: OTA/XYZ P2: ABC JWST082-FM JWST082-Kavanagh July 11, 2011 19:19 Printer Name: Yet to Come
P1: OTA/XYZ P2: ABC JWST082-FM JWST082-Kavanagh July 11, 2011 19:19 Printer Name: Yet to Come Fungi P1: OTA/XYZ P2: ABC JWST082-FM JWST082-Kavanagh July 11, 2011 19:19 Printer Name: Yet to Come Fungi Biology and Applications Second Edition Editor Kevin Kavanagh Department of Biology National University of Ireland Maynooth Maynooth County Kildare Ireland A John Wiley & Sons, Ltd., Publication P1: OTA/XYZ P2: ABC JWST082-FM JWST082-Kavanagh July 11, 2011 19:19 Printer Name: Yet to Come This edition first published 2011 © 2011 by John Wiley & Sons, Ltd. Wiley-Blackwell is an imprint of John Wiley & Sons, formed by the merger of Wiley’s global Scientific, Technical and Medical business with Blackwell Publishing. Registered Office: John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices: 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030-5774, USA For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/ wiley-blackwell. The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. -

Unexpected Cell Wall Alteration-Mediated Bactericidal
Unexpected cell wall alteration-mediated bactericidal activity of the antifungal caspofungin against vancomycin-resistant Enterococcus faecium Christophe Isnard, Sara B. Hernández, François Guérin, Fanny Joalland, Didier Goux, François Gravey, Michel Auzou, David P. Enot, Pierrick Meignen, Jean-Christophe Giard, et al. To cite this version: Christophe Isnard, Sara B. Hernández, François Guérin, Fanny Joalland, Didier Goux, et al.. Un- expected cell wall alteration-mediated bactericidal activity of the antifungal caspofungin against vancomycin-resistant Enterococcus faecium. Antimicrobial Agents and Chemotherapy, American So- ciety for Microbiology, 2020, 64 (10), 10.1128/AAC.01261-20. hal-02930693 HAL Id: hal-02930693 https://hal.archives-ouvertes.fr/hal-02930693 Submitted on 15 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Unexpected cell wall alteration-mediated bactericidal activity of the 2 antifungal caspofungin against vancomycin-resistant Enterococcus faecium 3 4 Running title: In vitro activity of caspofungin against E. faecium -

Effectiveness of 7.5 Percent Povidone Iodine in Comparison to 1 Percent Clotrimazole in the Treatment of Otomycosis
EFFECTIVENESS OF 7.5 PERCENT POVIDONE IODINE IN COMPARISON TO 1 PERCENT CLOTRIMAZOLE IN THE TREATMENT OF OTOMYCOSIS A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF M.S BRANCH –IV (OTORHINOLARYNGOLOGY EXAMINATION OF THE DR.MGR. MEDICAL UNIVERSITY TO BE HELD IN APRIL 2012 ACKNOWLEDGEMENTS I wish to express my deep gratitude to Dr Anand Job, Professor and Head of Unit 1, Department of Otorhinolaryngology, Speech and Hearing, Christian Medical College and Hospital, Vellore for his able guidance and encouragement in conducting this study and preparing this dissertation. I wish to express my deep gratitude to Dr Achamma Balraj, Head of the Department of Otorhinolaryngology, Speech and Hearing, Christian Medical College and Hospital, Vellore for her able guidance and encouragement in conducting this study and preparing this dissertation. I would like to thank Dr Rita Ruby Albert, Dr Regi Thomas, and Dr Rajan Sundaresan from the Department of Otorhinolaryngology for being my co-investigators in this study. I am extremely thankful to Dr Shalini Anandan, Assistant professor, Department of Microbiology for her guidance in this study. I am thankful to Dr Selvaraj from the Department of Biostatistics for his able guidance in the statistical analysis of this study. I would like to thank the Fluid Research Committee, CMC Hospital for granting me financial assistance for conducting this study. Last but not the least; I would like to thank all my patients who participated with me in this study for their kind co-operation. CERTIFICATE This is to certify that the dissertation entitled “Effectiveness of 7.5 percent povidone iodine in comparison to 1 percent clotrimazole in the treatment of otomycosis” is a bonafide original work of Dr Ajay Philip, submitted in partial fulfillment of the rules and regulations for the MS Branch IV, Otorhinolaryngology examination of The Tamil Nadu Dr. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -
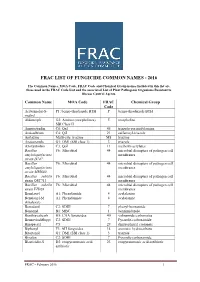
Frac List of Fungicide Common Names - 2016
FRAC LIST OF FUNGICIDE COMMON NAMES - 2016 The Common Names, MOA Code, FRAC Code and Chemical Group names included in this list are those used in the FRAC Code List and the associated List of Plant Pathogenic Organisms Resistant to Disease Control Agents. Common Name MOA Code FRAC Chemical Group Code Acibenzolar-S- P1: benzo-thiadiazole BTH P benzo-thiadiazole BTH methyl Aldimorph G2: Amines (morpholines) 5 morpholine SBI Class II Ametoctradin C8: QxI 45 triazolo-pyrimidylamine Amisulbrom C4: QiI 21 sulfamoyl-triazole Anilazine Multi-site: triazine M8 triazine Azaconazole G1: DMI (SBI class 1) 3 triazole Azoxystrobin C3: QoI 11 methoxy-acrylates Bacillus F6: Microbial 44 microbial disrupters of pathogen cell amyloliquefaciens membranes strain D747 Bacillus F6: Microbial 44 microbial disrupters of pathogen cell amyloliquefaciens membranes strain MBI600 Bacillus subtilis F6: Microbial 44 microbial disrupters of pathogen cell strain QST713 membranes Bacillus subtilis F6: Microbial 44 microbial disrupters of pathogen cell strain FZB24 membranes Benalaxyl A1: Phenylamide 4 acylalanine Benalaxyl-M A1: Phenylamide 4 acylalanine (kiralaxyl) Benodanil C2: SDHI 7 phenyl-benzamide Benomyl B1: MBC 1 benzimidazole Benthiavalicarb H5: CAA fungicides 40 valinamide carbamates Benzovindiflupyr C2: SDHI 7 Pyrazole-carboxamide Binapacryl C5 29 dinitrophenyl crotonate Biphenyl F3: AH fungicides 14 aromatic hydrocarbons Bitertanol G1: DMI (SBI class 1) 3 triazole Bixafen C2: SDHI 7 Pyrazole-carboxamide Blasticidin-S D2: enopyranuronic acid 23 enopyranuronic -

View U.S. Patent Application Publication No. US-2020-0056988
1111111111111111 IIIIII IIIII 1111111111 11111 11111 111111111111111 IIIII IIIII IIIIII IIII 11111111 US 20200056988Al c19) United States c12) Patent Application Publication c10) Pub. No.: US 2020/0056988 Al Butz et al. (43) Pub. Date: Feb. 20, 2020 (54) METHOD OF DETERMINING THE Publication Classification EFFICACY OF ANTIMICROBIALS (51) Int. Cl. GOIN 2113504 (2006.01) (71) Applicant: Wisconsin Alumni Research GOIN 33/497 (2006.01) Foundation, Madison, WI (US) (52) U.S. Cl. CPC ....... GOIN 2113504 (2013.01); GOIN 33/497 (72) Inventors: Daniel Elmer Butz, Madison, WI (US); (2013.01) Ann P. O'Rourke, Madison, WI (US); Mark E. Cook, Madison, WI (US) (57) ABSTRACT A method of determining efficacy of an antimicrobial treat ment in a subject includes calculating a breath delta value (21) Appl. No.: 16/540,627 (BDV) for each of at least six breath samples acquired from the subject over a 24 hour period starting from when the subject has been administered the antimicrobial treatment, (22) Filed: Aug. 14, 2019 calculating a mean standard deviation of BDV (SD BDV) across the six or more breath samples; and determining that the antimicrobial treatment is effective when the SD BDV is Related U.S. Application Data less than or equal to 0.46, or determining that the antimi crobial treatment is ineffective when the SD BDV is greater (60) Provisional application No. 62/764,874, filed on Aug. than 0.46. Also included are methods of treating a subject in 16, 2018. need of antimicrobial treatment. r Ass~sse~.f~;·el;~!~i!lt;·~n~~·~1)···· i ,, --~----·--··--···············r··············•······-·······•_.•.•.•.•_.•.•.•.•_J________ ___.........,., ; Excluded (no:299) r- ➔@!~;;;:~=:· ~··· Consented for partidpatfon {ir=32) Wi!hdrawn: • Exdusionary Criteria (ri=3) • Withdrawn by lnve.stigator(n"'2) Analyzed {n:=27) , Excluded from analysis {inadequate breath samples} {n=7} • 20 Subjects Anaiyze<l: o 13 Male; 7 Femi.lie o 76.2% Caucasian: VU% African American: 9.5% Other o 19% 18-35 yfo; 33_3% 36-54 yfo; 33.3% 55-73 yfo: i4.3 74+ yto Patent Application Publication Feb. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic