Mammography 1999
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RADIOGRAPHY to Prepare Individuals to Become Registered Radiologic Technologists
RADIOGRAPHY To prepare individuals to become Registered Radiologic Technologists. THE WORKFORCE CAPITAL This two-year, advanced medical program trains students in radiography. Radiography uses radiation to produce images of tissues, organs, bones and vessels of the body. The radiographer is an essential member of the health care team who works in a variety of settings. Responsibili- ties include accurately positioning the patient, producing quality diagnostic images, maintaining equipment and keeping computerized records. This certificate program of specialized training focuses on each of these responsibilities. Graduates are eligible to apply for the national credential examination to become a registered technologist in radiography, RT(R). Contact Student Services for current tuition rates and enrollment information. 580.242.2750 Mission, Goals, and Student Learning Outcomes Program Effectiveness Data Radiography Program Guidelines (Policies and Procedures) “The programs at Autry prepare you for the workforce with no extra training needed after graduation.” – Kenedy S. autrytech.edu ENDLESS POSSIBILITIES 1201 West Willow | Enid, Oklahoma, 73703 | 580.242.2750 | autrytech.edu COURSE LENGTH Twenty-four-month daytime program î August-July î Monday-Friday Academic hours: 8:15am-3:45pm Clinical hours: Eight-hour shifts between 7am-5pm with some ADMISSION PROCEDURES evening assignments required Applicants should contact Student Services at Autry Technology Center to request an information/application packet. Applicants who have a completed application on file and who have met entrance requirements will be considered for the program. Meeting ADULT IN-DISTRICT COSTS the requirements does not guarantee admission to the program. Qualified applicants will be contacted for an interview, and class Year One: $2732 (Additional cost of books and supplies approx: $1820) selection will be determined by the admissions committee. -

ACR–SPR-STR Practice Parameter for the Performance of Chest Radiography
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice parameters and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice parameters and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice parameter and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review and approval. The practice parameters and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice parameter and technical standard by those entities not providing these services is not authorized. Revised 2017 (Resolution 2)* ACR–SPR–STR PRACTICE PARAMETER FOR THE PERFORMANCE OF CHEST RADIOGRAPHY PREAMBLE This document is an educational tool designed to assist practitioners in providing appropriate radiologic care for patients. Practice Parameters and Technical Standards are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care1. -

MRC Review of Positron Emission Tomography (PET) Within the Medical Imaging Research Landscape
MRC Review of Positron Emission Tomography (PET) within The Medical Imaging Research Landscape August 2017 Content 1 Introduction 3 2 The medical imaging research landscape in the UK 4 2.1 Magnetic resonance imaging (MRI) 4 2.2 PET, including PET-MRI 6 2.3 Magnetoencephalography 7 3 Scientific uses and demand for PET imaging 8 3.1 Clinical practice 8 3.2 Research use of PET 8 3.3 Demand for PET 10 4 Bottlenecks 11 4.1 Cost 11 4.2 Radiochemistry requirements 12 4.3 Capacity 13 4.4 Analysis and modelling 13 5 Future Opportunities 14 5.1 Mitigating the high costs 14 5.2 Capacity building 14 5.3 Better Networking 15 6 Discussion and conclusions 16 Appendix 1 Experts consulted in the review 17 Appendix 2 Interests of other funders 18 Appendix 3 Usage and cost of PET in research 21 Appendix 4 Summary of facilities and capabilities across UK PET centres of excellence 23 2 1. Introduction This report aims to provide a review of Positron Emission Tomography (PET) within the medical imaging research landscape and a high level strategic review of the UK’s capabilities and needs in this area. The review was conducted by face-to-face and telephone interviews with 35 stakeholders from UK centres of excellence, international experts, industry and other funders (list at appendix 1). Data were also collected on facilities, resources and numbers of scans conducted across the centres of excellence using a questionnaire. The review has focused predominantly on PET imaging, but given MRC’s significant recent investment in other imaging modalities (7T Magnetic Resonance Imaging (MRI), hyperpolarised MRI) through the Clinical Research Infrastructure (CRI) Initiative, these are also considered more briefly. -

Breast Scintimammography
CLINICAL MEDICAL POLICY Policy Name: Breast Scintimammography Policy Number: MP-105-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 11/23/2020 Issue Date: 11/23/2020 Effective Date: 12/21/2020 Next Annual Review: 10/2021 Revision Date: 09/16/2020 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 5 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ does not provide coverage in the Company’s Medicaid products for breast scintimammography. The service is considered experimental and investigational in all applications, including but not limited to use as an adjunct to mammography or in staging the axillary lymph nodes. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-105-MD-PA Page 1 of 5 DEFINITIONS Prior Authorization Review Panel – A panel of representatives from within the Pennsylvania Department of Human Services who have been assigned organizational responsibility for the review, approval and denial of all PH-MCO Prior Authorization policies and procedures. Scintimammography A noninvasive supplemental diagnostic testing technology that requires the use of radiopharmaceuticals in order to detect tissues within the breast that accumulate higher levels of radioactive tracer that emit gamma radiation. -

Breast Imaging H
BREAST IMAGING H. Lee Moffitt Cancer Center and Research Institute Rotation Director: Margaret Szabunio, M.D. General Goals : On this rotation, the resident will learn to interpret screening mammograms and to perform diagnostic mammography and ultrasound examinations of the breast. The resident will learn to formulate appropriate differential diagnoses and recommendations for various breast pathologies. The resident will also learn mammographic, ultrasound and MR breast biopsy techniques. Daily Work : The resident rotation begins after morning conference has concluded. In this rotation the resident shall learn BIRADS nomenclature and become proficient in using the PENRAD system for reporting. The resident will also learn the difference between screening and diagnostic mammography and how to perform a diagnostic work-up. (S)he will become familiarized with mammographic positioning and technique and quality assurance including MQSA and ACR requirements. The resident will learn to interpret mammographic images and the use of additional mammographic views for problem solving. (S)he will learn when and how to employ sonography in patient evaluation. The resident is REQUIRED to attend Thursday morning breast interdisciplinary conference. Preparing and reviewing cases for this conference is highly recommended. The resident will assist with and perform needle localizations, breast biopsy and cyst aspiration procedures using mammographic, stereotactic and sonographic techniques for each. The resident is expected to identify proper indications and contraindications for each procedure and how to identify and manage complications. The resident is expected to understand and complete informed consent for image guided breast procedures. On occasion, the resident may observe or assist with ductography procedures. Opportunity to observe and assist with MR guided breast procedures may also be available. -

Surgical Options for Breast Cancer
The Breast Center Smilow Cancer Hospital 20 York Street, North Pavilion New Haven, CT 06510 Phone: (203) 200-2328 Fax: (203) 200-2075 SURGICAL OPTIONS There are a number of surgical procedures available today for the treatment of breast cancer. You will likely have a choice and will need to make your own decision, in consultation with your specific surgeon, about the best option for you. We offer you a choice because the research on the treatment of breast cancer has clearly shown that the cure and survival rates are the same regardless of what you choose. The choices can be divided into breast conserving options (i.e. lumpectomy or partial mastectomy) or breast removing options (mastectomy). A procedure to evaluate your armpit (axillary) lymph nodes will likely occur at the same time as your breast surgery. This is done to help determine the likelihood that cells from your breast cancer have left the breast and spread (metastasized) to another more dangerous location. This information will be used to help decide about your need for chemotherapy or hormone blocking drugs after surgery. PARTIAL MASTECTOMY (LUMPECTOMY) A partial mastectomy involves removing the cancer from your breast with a rim, or margin, of normal breast tissue. This allows the healthy noncancerous part of your breast to be preserved, and usually will not alter the sensation of the nipple. The benefit of this surgical choice is that it often preserves the cosmetics of the breast. Your surgeon will make a decision about the volume of tissue that needs removal in order to maximize the chance of clear margins as confirmed by our pathologist. -

Estimation of the Collective Effective Dose to the Population from Medical X-Ray Examinations in Finland
Estimation of the collective effective dose to the population from medical x-ray examinations in Finland Petra Tenkanen-Rautakoskia, Hannu Järvinena, Ritva Blya aRadiation and Nuclear Safety Authority (STUK), PL 14, 00880 Helsinki, Finland Abstract. The collective effective dose to the population from all x-ray examinations in Finland in 2005 was estimated. The numbers of x-ray examinations were collected by a questionnaire to the health care units (response rate 100 %). The effective doses in plain radiography were calculated using a Monte Carlo based program (PCXMC), as average values for selected health care units. For computed tomography (CT), weighted dose length product (DLPw) in a standard phantom was measured for routine CT protocols of four body regions, for 80 % of CT scanners including all types. The effective doses were calculated from DPLw values using published conversion factors. For contrast-enhanced radiology and interventional radiology, the effective dose was estimated mainly by using published DAP values and conversion factors for given body regions. About 733 examinations per 1000 inhabitants (excluding dental) were made in 2005, slightly less than in 2000. The proportions of plain radiography, computed tomography, contrast-enhanced radiography and interventional procedures were about 92, 7, 1 and 1 %, respectively. From 2000, the frequencies (number of examinations per 1000 inhabitants) of plain radiography and contrast-enhanced radiography have decreased about 8 and 33 %, respectively, while the frequencies of CT and interventional radiology have increased about 28 and 38 %, respectively. The population dose from all x-ray examinations is about 0,43 mSv per person (in 1997 0,5 mSv). -
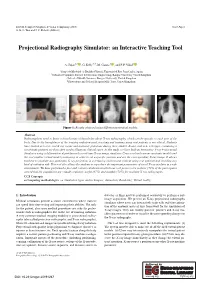
Projectional Radiography Simulator: an Interactive Teaching Tool
EG UK Computer Graphics & Visual Computing (2019) Short Paper G. K. L. Tam and J. C. Roberts (Editors) Projectional Radiography Simulator: an Interactive Teaching Tool A. Sujar1,2 , G. Kelly3,4, M. García1 , and F. P. Vidal2 1Grupo de Modelado y Realidad Virtual, Universidad Rey Juan Carlos, Spain 2School of Computer Science & Electronic Engineering, Bangor University United Kingdom 3School of Health Sciences, Bangor University, United Kingdom 4Shrewsbury and Telford Hospital NHS Trust, United Kingdom Figure 1: Results obtained using different anatomical models. Abstract Radiographers need to know a broad range of knowledge about X-ray radiography, which can be specific to each part of the body. Due to the harmfulness of the ionising radiation used, teaching and training using real patients is not ethical. Students have limited access to real X-ray rooms and anatomic phantoms during their studies. Books, and now web apps, containing a set of static pictures are then often used to illustrate clinical cases. In this study, we have built an Interactive X-ray Projectional Simulator using a deformation algorithm with a real-time X-ray image simulator. Users can load various anatomic models and the tool enables virtual model positioning in order to set a specific position and see the corresponding X-ray image. It allows teachers to simulate any particular X-ray projection in a lecturing environment without using real patients and avoiding any kind of radiation risk. This tool also allows the students to reproduce the important parameters of a real X-ray machine in a safe environment. We have performed a face and content validation in which our tool proves to be realistic (72% of the participants agreed that the simulations are visually realistic), useful (67%) and suitable (78%) for teaching X-ray radiography. -

Diagnostic Radiography Is the Production of High Quality Images for the Purpose of Diagnosis of Injury Or Disease
A Career in Medical Imaging What is Diagnostic Radiography / Medical Imaging? Diagnostic Radiography is the production of high quality images for the purpose of diagnosis of injury or disease. It is a pivotal aspect of medicine and a patient's diagnosis and ultimate treatment is often dependent on the images produced. Diagnostic Radiography uses both ionising and non-ionising radiation in the imaging process. The equipment used is at the high end of technology and computerisation within medicine. What does a Diagnostic Radiographer / Medical Imaging Technologist do? A Diagnostic Radiographer/Medical Imaging Technologist is a key member of the health care team. They are responsible for producing high quality medical images that assist medical specialists and practitioners to describe, diagnose, monitor and treat a patient’s injury or illness. Much of the medical equipment used to gain the images is highly technical and involves state of the art computerisation. A Diagnostic Radiographer/Medical Imaging Technologist needs to have the scientific and technological background to understand and use the equipment within a modern Radiology department as well as compassion and strong interpersonal skills. They need to be able to demonstrate care and understanding and have a genuine interest in a patient's welfare. The Diagnostic Radiographer/Medical Imaging Technologist will also need to be able to explain to the patient the need for the preparation and post examination care as well as the procedure to be undertaken. The Diagnostic Radiographer/Medical Imaging Technologist is able to work in a highly advanced technical profession that requires excellent people skills. It is an exciting and rewarding profession to embark on and great opportunities await the graduate. -

Therapeutic Mammaplasty Information for Patients the Aim of This Booklet Is to Give You Some General Information About Your Surgery
Oxford University Hospitals NHS Trust Therapeutic mammaplasty Information for patients The aim of this booklet is to give you some general information about your surgery. If you have any questions or concerns after reading it please discuss them with your breast care nurse practitioner or a member of staff at the Jane Ashley Centre. Telephone numbers are given at the end of this booklet. Author: Miss P.G.Roy, Consultant Oncoplastic Breast Surgeon Oxford University Hospitals NHS Trust Oxford OX3 9DU page 2 Therapeutic mammaplasty This operation involves combining a wide local excision (also known as a lumpectomy) with a breast reduction technique resulting in a smaller, uplifted and better shaped breast. This means that the lump can be removed with a wide rim of healthy tissue. The nipple and areola are preserved with their intact blood supply and the remaining breast tissue is repositioned to allow reshaping of the breast. The scars are either in the shape of a lollipop or an anchor (as shown below). You may have a drain placed in the wound to remove excess fluid; this is usually left in for 24 hours. This procedure can be carried out on one or both of your breasts, as discussed with your surgeon. Vertical mammaplasty Lollipop scar Wise pattern Anchor shaped scar mammaplasty page 3 Your nipple is moved to a new position to suit your new breast shape and size but it may end up in a position different to your wishes. The surgeon will try to achieve a mutually agreed breast size whilst performing the operation; however a cup size cannot be guaranteed and there are likely to be further significant changes to your breast after radiotherapy. -

Evaluation of Nipple Discharge
New 2016 American College of Radiology ACR Appropriateness Criteria® Evaluation of Nipple Discharge Variant 1: Physiologic nipple discharge. Female of any age. Initial imaging examination. Radiologic Procedure Rating Comments RRL* Mammography diagnostic 1 See references [2,4-7]. ☢☢ Digital breast tomosynthesis diagnostic 1 See references [2,4-7]. ☢☢ US breast 1 See references [2,4-7]. O MRI breast without and with IV contrast 1 See references [2,4-7]. O MRI breast without IV contrast 1 See references [2,4-7]. O FDG-PEM 1 See references [2,4-7]. ☢☢☢☢ Sestamibi MBI 1 See references [2,4-7]. ☢☢☢ Ductography 1 See references [2,4-7]. ☢☢ Image-guided core biopsy breast 1 See references [2,4-7]. Varies Image-guided fine needle aspiration breast 1 Varies *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Pathologic nipple discharge. Male or female 40 years of age or older. Initial imaging examination. Radiologic Procedure Rating Comments RRL* See references [3,6,8,10,13,14,16,25- Mammography diagnostic 9 29,32,34,42-44,71-73]. ☢☢ See references [3,6,8,10,13,14,16,25- Digital breast tomosynthesis diagnostic 9 29,32,34,42-44,71-73]. ☢☢ US is usually complementary to mammography. It can be an alternative to mammography if the patient had a recent US breast 9 mammogram or is pregnant. See O references [3,5,10,12,13,16,25,30,31,45- 49]. MRI breast without and with IV contrast 1 See references [3,8,23,24,35,46,51-55]. -

Therapeutic Mammoplasty
Therapeutic mammoplasty This information is for women undergoing a therapeutic mammoplasty and explains what happens during the operation, outlining the benefits, alternatives and risks of surgery. If there is anything that you do not understand or you have further questions or concerns please speak to one of the breast care nurses. Their contact details are listed at the end of this document. What is a therapeutic mammoplasty? Therapeutic mammoplasty is an operation to remove the breast cancer (therapeutic) and then reshape the breast by removing skin and breast tissue (mammoplasty), to try to preserve a normal breast shape that will usually be smaller and more uplifted. There is a limit to how much breast tissue can be removed with a standard lumpectomy without adversely affecting the appearance of the breast, but this technique allows us to remove more breast tissue and attempt to leave an acceptable cosmetic outcome. The operation is suitable for women with moderate to larger breasts, and who have a degree of droop (ptosis). If there is significant asymmetry (difference between your breasts) afterwards, the breast on the other side may also need to be reduced, to provide a better match in size and shape if so desired. This is known as symmetrisation surgery and will be performed at a later date. What are the advantages? • The technique aims to produce a normal breast shape with no indentation, distortion or loss of cleavage that might otherwise be likely. It is particularly useful for lower breast tumours that are more likely to develop a deformity if a simple lumpectomy is performed.