Zuo, H., Ueland, P. M., Midttun, Tell, G. S., Fanidi, A., Zheng, W., Shu, X., Xiang, Y., Wu, J., Prentice, R., Pettinger, M., Thomson, C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Animals and Morality Tales in Hayashi Razan's Kaidan Zensho
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses Dissertations and Theses March 2015 The Unnatural World: Animals and Morality Tales in Hayashi Razan's Kaidan Zensho Eric Fischbach University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/masters_theses_2 Part of the Chinese Studies Commons, Japanese Studies Commons, and the Translation Studies Commons Recommended Citation Fischbach, Eric, "The Unnatural World: Animals and Morality Tales in Hayashi Razan's Kaidan Zensho" (2015). Masters Theses. 146. https://doi.org/10.7275/6499369 https://scholarworks.umass.edu/masters_theses_2/146 This Open Access Thesis is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. THE UNNATURAL WORLD: ANIMALS AND MORALITY TALES IN HAYASHI RAZAN’S KAIDAN ZENSHO A Thesis Presented by ERIC D. FISCHBACH Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of MASTER OF ARTS February 2015 Asian Languages and Literatures - Japanese © Copyright by Eric D. Fischbach 2015 All Rights Reserved THE UNNATURAL WORLD: ANIMALS AND MORALITY TALES IN HAYASHI RAZAN’S KAIDAN ZENSHO A Thesis Presented by ERIC D. FISCHBACH Approved as to style and content by: __________________________________________ Amanda C. Seaman, Chair __________________________________________ Stephen Miller, Member ________________________________________ Stephen Miller, Program Head Asian Languages and Literatures ________________________________________ William Moebius, Department Head Languages, Literatures, and Cultures ACKNOWLEDGMENTS I would like to thank all my professors that helped me grow during my tenure as a graduate student here at UMass. -

Treating Osteoarthritis with Chinese Herbs by Jake Schmalzriedt, DOM
TREATING OSTEOARTHRITIS WITH CHINESE HERBS By Jake Schmalzriedt, DOM Osteoarthritis is a progressive joint disorder that is also known as WESTERN MEDICAL DIAGNOSIS degenerative joint disease, degenerative arthritis, osteoarthrosis Western diagnosis is made primarily from signs and symptoms, (implying lack of inflammation), and commonly “wear and tear” history, and a physical exam checking for tenderness, alignment, arthritis. It is the gradual breakdown of cartilage in the joints and gait, stability, range of motion, and absence of an inflammatory the development of bony spurs at the margins of the joints. The response (heat, redness, and swelling). Western blood work is term osteoarthritis is derived from the Greek words, osteo mean- also used to rule out rheumatoid arthritis and gout. X-rays can ing bone, arthro meaning joint, and itis referring to inflamma- show joint narrowing and osteophyte formation, confirming the tion. This is somewhat of a contradictory term as osteoarthritis osteoarthritis diagnosis. generally has little inflammation associated with it. WESTERN MEDICAL TREATMENT Osteoarthritis falls under rheumatic diseases. There are two main The Western medical treatment principle is categories of arthritis: inflammatory and non- Cartilage and symptomatic relief and supportive therapy inflammatory. Osteoarthritis belongs in the Bone Fragment Normal Bone with an emphasis on controlling pain, in- non-inflammatory category. There are over Thinned Cartilage creasing function and range of motion, and 100 different types of arthritis (all sharing the Normal Cartilage improving quality of life. common symptom of persistent joint pain) Eroded Cartilage with osteoarthritis being the most common Western Therapy and affecting over 27 million people in the Physical therapy and gentle exercises are United States. -

Is Shuma the Chinese Analog of Soma/Haoma? a Study of Early Contacts Between Indo-Iranians and Chinese
SINO-PLATONIC PAPERS Number 216 October, 2011 Is Shuma the Chinese Analog of Soma/Haoma? A Study of Early Contacts between Indo-Iranians and Chinese by ZHANG He Victor H. Mair, Editor Sino-Platonic Papers Department of East Asian Languages and Civilizations University of Pennsylvania Philadelphia, PA 19104-6305 USA [email protected] www.sino-platonic.org SINO-PLATONIC PAPERS FOUNDED 1986 Editor-in-Chief VICTOR H. MAIR Associate Editors PAULA ROBERTS MARK SWOFFORD ISSN 2157-9679 (print) 2157-9687 (online) SINO-PLATONIC PAPERS is an occasional series dedicated to making available to specialists and the interested public the results of research that, because of its unconventional or controversial nature, might otherwise go unpublished. The editor-in-chief actively encourages younger, not yet well established, scholars and independent authors to submit manuscripts for consideration. Contributions in any of the major scholarly languages of the world, including romanized modern standard Mandarin (MSM) and Japanese, are acceptable. In special circumstances, papers written in one of the Sinitic topolects (fangyan) may be considered for publication. Although the chief focus of Sino-Platonic Papers is on the intercultural relations of China with other peoples, challenging and creative studies on a wide variety of philological subjects will be entertained. This series is not the place for safe, sober, and stodgy presentations. Sino- Platonic Papers prefers lively work that, while taking reasonable risks to advance the field, capitalizes on brilliant new insights into the development of civilization. Submissions are regularly sent out to be refereed, and extensive editorial suggestions for revision may be offered. Sino-Platonic Papers emphasizes substance over form. -
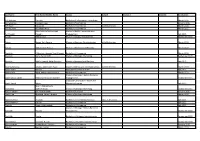
Last Name First Name/Middle Name Course Award Course 2 Award 2 Graduation
Last Name First Name/Middle Name Course Award Course 2 Award 2 Graduation A/L Krishnan Thiinash Bachelor of Information Technology March 2015 A/L Selvaraju Theeban Raju Bachelor of Commerce January 2015 A/P Balan Durgarani Bachelor of Commerce with Distinction March 2015 A/P Rajaram Koushalya Priya Bachelor of Commerce March 2015 Hiba Mohsin Mohammed Master of Health Leadership and Aal-Yaseen Hussein Management July 2015 Aamer Muhammad Master of Quality Management September 2015 Abbas Hanaa Safy Seyam Master of Business Administration with Distinction March 2015 Abbasi Muhammad Hamza Master of International Business March 2015 Abdallah AlMustafa Hussein Saad Elsayed Bachelor of Commerce March 2015 Abdallah Asma Samir Lutfi Master of Strategic Marketing September 2015 Abdallah Moh'd Jawdat Abdel Rahman Master of International Business July 2015 AbdelAaty Mosa Amany Abdelkader Saad Master of Media and Communications with Distinction March 2015 Abdel-Karim Mervat Graduate Diploma in TESOL July 2015 Abdelmalik Mark Maher Abdelmesseh Bachelor of Commerce March 2015 Master of Strategic Human Resource Abdelrahman Abdo Mohammed Talat Abdelziz Management September 2015 Graduate Certificate in Health and Abdel-Sayed Mario Physical Education July 2015 Sherif Ahmed Fathy AbdRabou Abdelmohsen Master of Strategic Marketing September 2015 Abdul Hakeem Siti Fatimah Binte Bachelor of Science January 2015 Abdul Haq Shaddad Yousef Ibrahim Master of Strategic Marketing March 2015 Abdul Rahman Al Jabier Bachelor of Engineering Honours Class II, Division 1 -

Ch'uan Shu Tsung Mu, Ch
F '£2i)l,(: j{, :;i/(r;l£'fili w,,, \l0 t 11<,,,,.,;,s-1y:.tls\ '"'%•,:5 ,\f�i)�frIS ·!M . '.� ' , \,jf:); 'ii• • " &i >�,, i - ., .., ;;. · .... - .. ERR AT.A Page 5: Line 11, fill in characters for Che-tung it� Az . ,Linen·12, after the·word "written",. .fill inih}Jl . .Line 15,· . after the ·word "wr.i te� ", , fill· in .,fN . .Line 31 should. read,. "Comment: we·hold·.that rl y·ang· ip, written .f MAN SHU Book of the southern Barbarians THE CORNELL UNIVERSITY SOUTHEAST ASIA PROGRAM The Southeast Asia Program was organized at Cornell University in the Department of Far Eastern Studies in 1950. It is a teaching and research program of inter- · disciplinary studies in the humanities, social sciences, and some natural sciences. It deals with Southeast Asia as a region, and with the individual countries of the area: Burma, Cambodia, Indonesia, Laos, Malaya, the Philippines, Thailand, and Vietnam. The activities of the Program are carried on both at Cornell and in southeast Asia. They include an under graduate and graduate curriculum at Cornell which provides instruction by special.ists in southeast Asian cultural history and present-day aff�irs and offers intensive train� ing in each of the major languages of the area. The Program sponsors group research projects on Thailand, on Indonesia, on the Philippines, and on the area's Chinese minorities. At the same time, individual staff and students of the Program have done field research in every Southeast Asian country• . A list of publications relating to Southeast Asia which may be obtained on prepaid order directly from the Program is given at the end of this volume. -

Chinese Language Resources Cataloging Priorities and Status
Table 1/2: Cataloging priorities and status for databases/packages that can be cataloged at title-level Prioritized according to UC East Asian bibliographers’ decision made in July 2009; status updated on April 19, 2010 Priority T and/or i Subscribing Resourc Title list/Vendor Records Proposed Package Name Summary/OCLC Records/OpenURLs Current e Campuses e Type Timeline/ Status r Taiwan Electronic Periodicals Title list available by request but not Services (TEPS) = *700 titles (including hidden titles), 280 available online, no new titles/coverage Done in July 台灣電子期刊服 alerts; rich metadata, one clear (zoom-in Ongoing existing OCLC records (~40% hit rate), 420 k- 2008 with 務網 2 all Journals feature) cover image of some random maintenance (Taiwan dian level records the title list zi qi kan fu wu issue posted, title changes listed; 10% *Not included in SFX KB, using PID of Apr. 2008 wang) incorrect ISSNs; 40% have incorrect http://uclibs.org/P imprint info. for the first issue ID/126969 *3400 titles, 400 new titles from Aug. 2008 China Academic title list (initial list: 2007 Dec. list), 600+ hidden Journals (CAJ) = 中 titles Title lists available on Web site, but no 国期刊全文数据 *3200 existing OCLC records (97% hit rate due new titles/coverage alerts; one blur cover Done in May Ongoing 库 (Zhongguo qi to Univ. of Hong Kong’s loading CAJ as image of some random issue posted, no 2009 with 1 all Journals maintenance kan quan wen shu Collection Sets recently, with quite a few title page/back cover/TOC/advertisement the title list ju ku) hybrid records,), 75 k-level records images, hidden title changes; 9.7% of Dec. -

Linguistic Composition and Characteristics of Chinese Given Names DOI: 10.34158/ONOMA.51/2016/8
Onoma 51 Journal of the International Council of Onomastic Sciences ISSN: 0078-463X; e-ISSN: 1783-1644 Journal homepage: https://onomajournal.org/ Linguistic composition and characteristics of Chinese given names DOI: 10.34158/ONOMA.51/2016/8 Irena Kałużyńska Sinology Department Faculty of Oriental Studies University of Warsaw e-mail: [email protected] To cite this article: Kałużyńska, Irena. 2016. Linguistic composition and characteristics of Chinese given names. Onoma 51, 161–186. DOI: 10.34158/ONOMA.51/2016/8 To link to this article: https://doi.org/10.34158/ONOMA.51/2016/8 © Onoma and the author. Linguistic composition and characteristics of Chinese given names Abstract: The aim of this paper is to discuss various linguistic and cultural aspect of personal naming in China. In Chinese civilization, personal names, especially given names, were considered crucial for a person’s fate and achievements. The more important the position of a person, the more various categories of names the person received. Chinese naming practices do not restrict the inventory of possible given names, i.e. given names are formed individually, mainly as a result of a process of onymisation, and given names are predominantly semantically transparent. Therefore, given names seem to be well suited for a study of stereotyped cultural expectations present in Chinese society. The paper deals with numerous subdivisions within the superordinate category of personal name, as the subclasses of surname and given name. It presents various subcategories of names that have been used throughout Chinese history, their linguistic characteristics, their period of origin, and their cultural or social functions. -

The Muslim Emperor of China: Everyday Politics in Colonial Xinjiang, 1877-1933
The Muslim Emperor of China: Everyday Politics in Colonial Xinjiang, 1877-1933 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Schluessel, Eric T. 2016. The Muslim Emperor of China: Everyday Politics in Colonial Xinjiang, 1877-1933. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:33493602 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA The Muslim Emperor of China: Everyday Politics in Colonial Xinjiang, 1877-1933 A dissertation presented by Eric Tanner Schluessel to The Committee on History and East Asian Languages in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of History and East Asian Languages Harvard University Cambridge, Massachusetts April, 2016 © 2016 – Eric Schluessel All rights reserved. Dissertation Advisor: Mark C. Elliott Eric Tanner Schluessel The Muslim Emperor of China: Everyday Politics in Colonial Xinjiang, 1877-1933 Abstract This dissertation concerns the ways in which a Chinese civilizing project intervened powerfully in cultural and social change in the Muslim-majority region of Xinjiang from the 1870s through the 1930s. I demonstrate that the efforts of officials following an ideology of domination and transformation rooted in the Chinese Classics changed the ways that people associated with each other and defined themselves and how Muslims understood their place in history and in global space. -

Names of Chinese People in Singapore
101 Lodz Papers in Pragmatics 7.1 (2011): 101-133 DOI: 10.2478/v10016-011-0005-6 Lee Cher Leng Department of Chinese Studies, National University of Singapore ETHNOGRAPHY OF SINGAPORE CHINESE NAMES: RACE, RELIGION, AND REPRESENTATION Abstract Singapore Chinese is part of the Chinese Diaspora.This research shows how Singapore Chinese names reflect the Chinese naming tradition of surnames and generation names, as well as Straits Chinese influence. The names also reflect the beliefs and religion of Singapore Chinese. More significantly, a change of identity and representation is reflected in the names of earlier settlers and Singapore Chinese today. This paper aims to show the general naming traditions of Chinese in Singapore as well as a change in ideology and trends due to globalization. Keywords Singapore, Chinese, names, identity, beliefs, globalization. 1. Introduction When parents choose a name for a child, the name necessarily reflects their thoughts and aspirations with regards to the child. These thoughts and aspirations are shaped by the historical, social, cultural or spiritual setting of the time and place they are living in whether or not they are aware of them. Thus, the study of names is an important window through which one could view how these parents prefer their children to be perceived by society at large, according to the identities, roles, values, hierarchies or expectations constructed within a social space. Goodenough explains this culturally driven context of names and naming practices: Department of Chinese Studies, National University of Singapore The Shaw Foundation Building, Block AS7, Level 5 5 Arts Link, Singapore 117570 e-mail: [email protected] 102 Lee Cher Leng Ethnography of Singapore Chinese Names: Race, Religion, and Representation Different naming and address customs necessarily select different things about the self for communication and consequent emphasis. -

The Acquisition of Mandarin Prosody by American Learners of Chinese As a Foreign Language
The Acquisition of Mandarin Prosody by American Learners of Chinese as a Foreign Language (CFL) Dissertation Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By Chunsheng Yang, B.A., M.A. Graduate Program in East Asian Languages and Literatures The Ohio State University 2011 Dissertation Committee: Dr. Marjorie K.M. Chan, Advisor Dr. Mary E. Beckman Dr. Cynthia Clopper Dr. Mineharu Nakayama Copyright by Chunsheng Yang 2011 ABSTRACT In the acquisition of second language (L2) or foreign language (FL) pronunciation, learners not only learn how to pronounce consonants and vowels (tones as well, in the case of tone languages, such as Mandarin Chinese), they also learn how to produce the vowel reduction, vowel-consonant co-articulation, and prosody. Central to this dissertation is prosody, which refers to the way that an utterance is broken up into smaller units, and the acoustic patterns of each unit at different levels, in terms of fundamental frequency (F0), duration and amplitude. In L2 pronunciation, prosody is as important as -- if not more important than -- consonants and vowels. This dissertation examines the acquisition of Mandarin prosody by American learners of Chinese as a Foreign Language (CFL). Specifically, it examines four aspects of Mandarin prosody: (1) prosodic phrasing (i.e., breaking up of utterances into smaller units); (2) surface F0 and duration patterns of prosodic phrasing in a group of sentence productions elicited from L1 and L2 speakers of Mandarin Chinese; (3) patterns of tones errors in L2 Mandarin productions; and (4) the relationship between tone errors and prosodic phrasing in L2 Mandarin. -

Surname Methodology in Defining Ethnic Populations : Chinese
Surname Methodology in Defining Ethnic Populations: Chinese Canadians Ethnic Surveillance Series #1 August, 2005 Surveillance Methodology, Health Surveillance, Public Health Division, Alberta Health and Wellness For more information contact: Health Surveillance Alberta Health and Wellness 24th Floor, TELUS Plaza North Tower P.O. Box 1360 10025 Jasper Avenue, STN Main Edmonton, Alberta T5J 2N3 Phone: (780) 427-4518 Fax: (780) 427-1470 Website: www.health.gov.ab.ca ISBN (on-line PDF version): 0-7785-3471-5 Acknowledgements This report was written by Dr. Hude Quan, University of Calgary Dr. Donald Schopflocher, Alberta Health and Wellness Dr. Fu-Lin Wang, Alberta Health and Wellness (Authors are ordered by alphabetic order of surname). The authors gratefully acknowledge the surname review panel members of Thu Ha Nguyen and Siu Yu, and valuable comments from Yan Jin and Shaun Malo of Alberta Health & Wellness. They also thank Dr. Carolyn De Coster who helped with the writing and editing of the report. Thanks to Fraser Noseworthy for assisting with the cover page design. i EXECUTIVE SUMMARY A Chinese surname list to define Chinese ethnicity was developed through literature review, a panel review, and a telephone survey of a randomly selected sample in Calgary. It was validated with the Canadian Community Health Survey (CCHS). Results show that the proportion who self-reported as Chinese has high agreement with the proportion identified by the surname list in the CCHS. The surname list was applied to the Alberta Health Insurance Plan registry database to define the Chinese ethnic population, and to the Vital Statistics Death Registry to assess the Chinese ethnic population mortality in Alberta. -

Remaking History: the Shu and Wu Perspectives in the Three Kingdoms Period
Remaking History: The Shu and Wu Perspectives in the Three Kingdoms Period XIAOFEI TIAN HARVARD UNIVERSITY Of the three powers—Wei, Shu, and Wu—that divided China for the better part of the third century, Wei has received the most attention in the standard literary historical accounts. In a typical book of Chinese literary history in any language, little, if anything, is said about Wu and Shu. This article argues that the consider- ation of the literary production of Shu and Wu is crucial to a fuller picture of the cultural dynamics of the Three Kingdoms period. The three states competed with one another for the claim to political legitimacy and cultural supremacy, and Wu in particular was in a position to contend with Wei in its cultural undertakings, notably in the areas of history writing and ritual music. This article begins with an overview of Shu and Wu literary production, and moves on to a more detailed discussion of Wu’s cultural projects, both of which were intended to assert Wu’s legitimacy and cultural power vis-à-vis Wei and Shu’s claims to cultural and polit- ical orthodoxy. Ultimately, this article implicitly asks the question of how to write literary history when there is scant material from the period under question, and suggests that we perform textual excavations and make use of what we have to try and reconstruct, as best as we can, what once was. A good literary history of the Chinese medieval period, the age of manuscript culture and that of heavy textual losses and transfigurations, should be written with the awareness of the incomplete and imperfect nature of the data we do have, and incorporate the phenomenon of textual losses and transfigurations as well as some reflections on the underlying reasons into its narrative and critical inquiry.