Large Mesozoic Mammals Fed on Young Dinosaurs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mesozoic—Dinos!
MESOZOIC—DINOS! VOLUME 9, ISSUE 8, APRIL 2020 THIS MONTH DINOSAURS! • Dinosaurs ○ What is a Dinosaur? page 2 DINOSAURS! When people think paleontology, ○ Bird / Lizard Hip? page 5 they think of scientists ○ Size Activity 1 page 10 working in the hot sun of ○ Size Activity 2 page 13 Colorado National ○ Size Activity 3 page 43 Monument or the Badlands ○ Diet page 46 of South Dakota and ○ Trackways page 53 Wyoming finding enormous, ○ Colorado Fossils and fierce, and long-gone Dinosaurs page 66 dinosaurs. POWER WORDS Dinosaurs safely evoke • articulated: fossil terror. Better than any bones arranged in scary movie, these were Articulated skeleton of the Tyrannosaurus rex proper order actually living breathing • endothermic: an beasts! from the American Museum of Natural History organism produces body heat through What was the biggest dinosaur? be reviewing the information metabolism What was the smallest about dinosaurs, but there is an • metabolism: chemical dinosaur? What color were interview with him at the end of processes that occur they? Did they live in herds? this issue. Meeting him, you will within a living organism What can their skeletons tell us? know instantly that he loves his in order to maintain life What evidence is there so that job! It doesn’t matter if you we can understand more about become an electrician, auto CAREER CONNECTION how these animals lived. Are mechanic, dancer, computer • Meet Dr. Holtz, any still alive today? programmer, author, or Dinosaur paleontologist, I truly hope that Paleontologist! page 73 To help us really understand you have tremendous job more about dinosaurs, we have satisfaction, like Dr. -

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

The Geologic Time Scale Is the Eon
Exploring Geologic Time Poster Illustrated Teacher's Guide #35-1145 Paper #35-1146 Laminated Background Geologic Time Scale Basics The history of the Earth covers a vast expanse of time, so scientists divide it into smaller sections that are associ- ated with particular events that have occurred in the past.The approximate time range of each time span is shown on the poster.The largest time span of the geologic time scale is the eon. It is an indefinitely long period of time that contains at least two eras. Geologic time is divided into two eons.The more ancient eon is called the Precambrian, and the more recent is the Phanerozoic. Each eon is subdivided into smaller spans called eras.The Precambrian eon is divided from most ancient into the Hadean era, Archean era, and Proterozoic era. See Figure 1. Precambrian Eon Proterozoic Era 2500 - 550 million years ago Archaean Era 3800 - 2500 million years ago Hadean Era 4600 - 3800 million years ago Figure 1. Eras of the Precambrian Eon Single-celled and simple multicelled organisms first developed during the Precambrian eon. There are many fos- sils from this time because the sea-dwelling creatures were trapped in sediments and preserved. The Phanerozoic eon is subdivided into three eras – the Paleozoic era, Mesozoic era, and Cenozoic era. An era is often divided into several smaller time spans called periods. For example, the Paleozoic era is divided into the Cambrian, Ordovician, Silurian, Devonian, Carboniferous,and Permian periods. Paleozoic Era Permian Period 300 - 250 million years ago Carboniferous Period 350 - 300 million years ago Devonian Period 400 - 350 million years ago Silurian Period 450 - 400 million years ago Ordovician Period 500 - 450 million years ago Cambrian Period 550 - 500 million years ago Figure 2. -

Recent Advances in Studies on Mesozoic and Paleogene Mammals in China
Vol.24 No.2 2010 Paleomammalogy Recent Advances in Studies on Mesozoic and Paleogene Mammals in China WANG Yuanqing* and NI Xijun Institute of Vertebrate Paleontology and Paleoanthropology, CAS, Beijing 10004, China ike in other fields of paleontology, research in from the articular of the lower jaw and the quadrate of the paleomammalogy mainly falls into two aspects. cranium following the process of reduction and detachment LOne is related to the biological nature of fossil from the reptilian mandible, are new elements of the bony mammals, such as their systematics, origin, evolution, chain in the mammalian middle ear. The appearance of phylogenetic relationships, transformation of key features mammalian middle ear allows mammals to hear the sound and paleobiogeography, and the other is related to the of higher frequencies than reptiles do. Generally speaking, geological context, involving biostratigraphy, biochronology, a widely accepted hypothesis is that mammals originated faunal turnover and its relations to the global and regional from a certain extinct reptilian group. The formation and environmental changes. In the last decade, a number of development of the definitive mammalian middle ear well-preserved mammalian specimens were collected from (DMME) has thus become one of the key issues in the study different localities around the country. Such discoveries have of mammalian evolution and has drawn the attention from provided significant information for understanding the early many researchers for many years. evolution of mammals and reconstructing the phylogeny of Developmental biological studies have proven the early mammals. function of the Meckel’s cartilage and its relationship to Studies of Chinese Mesozoic mammals achieved the ear ossicles during the development of mammalian remarkable progress in the past several years. -

Uncorking the Bottle: What Triggered the Paleocene/Eocene Thermal Maximum Methane Release? Miriame
PALEOCEANOGRAPHY, VOL. 16, NO. 6, PAGES 549-562, DECEMBER 2001 Uncorking the bottle: What triggered the Paleocene/Eocene thermal maximum methane release? MiriamE. Katz,• BenjaminS. Cramer,Gregory S. Mountain,2 Samuel Katz, 3 and KennethG. Miller,1,2 Abstract. The Paleocene/Eocenethermal maximum (PETM) was a time of rapid global warming in both marine and continentalrealms that has been attributed to a massivemethane (CH4) releasefrom marine gas hydrate reservoirs. Previously proposedmechanisms for thismethane release rely on a changein deepwatersource region(s) to increasewater temperatures rapidly enoughto trigger the massivethermal dissociationof gas hydratereservoirs beneath the seafloor.To establish constraintson thermaldissociation, we modelheat flow throughthe sedimentcolumn and showthe effectof the temperature changeon the gashydrate stability zone throughtime. In addition,we provideseismic evidence tied to boreholedata for methanerelease along portions of the U.S. continentalslope; the releasesites are proximalto a buriedMesozoic reef front. Our modelresults, release site locations, published isotopic records, and oceancirculation models neither confirm nor refute thermaldissociation as the triggerfor the PETM methanerelease. In the absenceof definitiveevidence to confirmthermal dissociation,we investigatean altemativehypothesis in which continentalslope failure resulted in a catastrophicmethane release.Seismic and isotopic evidence indicates that Antarctic source deepwater circulation and seafloor erosion caused slope retreatalong -

Mammals from the Mesozoic of Mongolia
Mammals from the Mesozoic of Mongolia Introduction and Simpson (1926) dcscrihed these as placental (eutherian) insectivores. 'l'he deltathcroids originally Mongolia produces one of the world's most extraordi- included with the insectivores, more recently have narily preserved assemblages of hlesozoic ma~nmals. t)een assigned to the Metatheria (Kielan-Jaworowska Unlike fossils at most Mesozoic sites, Inany of these and Nesov, 1990). For ahout 40 years these were the remains are skulls, and in some cases these are asso- only Mesozoic ~nanimalsknown from Mongolia. ciated with postcranial skeletons. Ry contrast, 'I'he next discoveries in Mongolia were made by the Mesozoic mammals at well-known sites in North Polish-Mongolian Palaeontological Expeditions America and other continents have produced less (1963-1971) initially led by Naydin Dovchin, then by complete material, usually incomplete jaws with den- Rinchen Barsbold on the Mongolian side, and Zofia titions, or isolated teeth. In addition to the rich Kielan-Jaworowska on the Polish side, Kazi~nierz samples of skulls and skeletons representing Late Koualski led the expedition in 1964. Late Cretaceous Cretaceous mam~nals,certain localities in Mongolia ma~nmalswere collected in three Gohi Desert regions: are also known for less well preserved, but important, Bayan Zag (Djadokhta Formation), Nenlegt and remains of Early Cretaceous mammals. The mammals Khulsan in the Nemegt Valley (Baruungoyot from hoth Early and Late Cretaceous intervals have Formation), and llcrmiin 'ISav, south-\vest of the increased our understanding of diversification and Neniegt Valley, in the Red beds of Hermiin 'rsav, morphologic variation in archaic mammals. which have heen regarded as a stratigraphic ecluivalent Potentially this new information has hearing on the of the Baruungoyot Formation (Gradzinslti r't crl., phylogenetic relationships among major branches of 1977). -

The Triassic Period and the Beginning of the Mesozoic Era
Readings and Notes An Introduction to Earth Science 2016 The Triassic Period and the Beginning of the Mesozoic Era John J. Renton Thomas Repine Follow this and additional works at: https://researchrepository.wvu.edu/earthscience_readings Part of the Geology Commons C\.\- \~ THE TRIASSIC PERIOD and the BEGINNING OF THE MESOZOIC ERA Introduction to the Mesozoic Era: The Triassic Period is the first period of the Mesozoic Era, a span of time from 245 million years ago to 66 million years ago. Although the Mesozoic era commonly known as the "Age of the Dinosaurs", it should be pointed out that there were other important evolutionary developments taking place such as the appearance of the first mammal birds and flowering plans. The onset of the Mesozoic Era, the Triassic Period, was also a time of profound tectonic activity affecting the entire North American craton. In the east, the primary event was the breakup of Pangea and the formation of the Atlantic Ocean. In the west, it was the formation ofan Andean-type continental margin as the newly-formed continent of North America rapidly moved westward in response to the opening of the Atlantic Ocean coupled with the addition of exotic terranes to the western margin of the continent.. As the Atlantic oceanic ridge rose, the volume of ocean waters that was displaced was sufficient to result in the most extensive flooding of the continent by an epeiric sea since the Paleozoic; a sea whose presence was recorded by the accumulation of extensive carbonates throughout the continental interior. In the oceans, new life forms evolved to fill the vacancies brought about by the Permian extinction. -

Mammal Evolution
Mammal Evolution Geology 331 Paleontology Triassic synapsid reptiles: Therapsids or mammal-like reptiles. Note the sprawling posture. Mammal with Upright Posture From Synapsids to Mammals, a well documented transition series Carl Buell Prothero, 2007 Synapsid Teeth, less specialized Mammal Teeth, more specialized Prothero, 2007 Yanoconodon, Lower Cretaceous of China Yanoconodon, Lower Cretaceous of China, retains ear bones attached to the inside lower jaw Morganucodon Yanoconodon = articular of = quadrate of Human Ear Bones, or lower reptile upper reptile Auditory Ossicles jaw jaw Cochlea Mammals have a bony secondary palate Primary Palate Reptiles have a soft Secondary Palate secondary palate Reduction of digit bones from Hand and Foot of Permian Synapsid 2-3-4-5-3 in synapsid Seymouria ancestors to 2-3-3-3-3 in mammals Human Hand and Foot Class Mammalia - Late Triassic to Recent Superorder Tricodonta - Late Triassic to Late Cretaceous Superorder Multituberculata - Late Jurassic to Early Oligocene Superorder Monotremata - Early Cretaceous to Recent Superorder Metatheria (Marsupials) - Late Cretaceous to Recent Superorder Eutheria (Placentals) - Late Cretaceous to Recent Evolution of Mammalian Superorders Multituberculates Metatheria Eutheria (Marsupials) (Placentals) Tricodonts Monotremes . Live Birth Extinct: . .. Mammary Glands? Mammals in the Age of Dinosaurs – a nocturnal life style Hadrocodium, a lower Jurassic mammal with a “large” brain (6 mm brain case in an 8 mm skull) Were larger brains adaptive for a greater sense of smell? Big Brains and Early Mammals July 14, 2011 The Academic Minute http://www.insidehighered.com/audio/academic_pulse/big_brains_and_early_mammals Lower Cretaceous mammal from China Jawbones of a Cretaceous marsupial from Mongolia Mammal fossil from the Cretaceous of Mongolia Reconstructed Cretaceous Mammal Early Cretaceous mammal ate small dinosaurs Repenomamus robustus fed on psittacosaurs. -

Early Cretaceous Amphilestid ('Triconodont') Mammals from Mongolia
Early Cretaceous amphilestid ('triconodont') mammals from Mongolia ZOFIAKIELAN-JAWOROWSKA and DEMBERLYIN DASHZEVEG Kielan-Jaworowską Z. &Daslueveg, D. 1998. Early Cretaceous amphilestid (.tricono- dont') mammals from Mongotia. - Acta Pal.aeontol.ogicaPolonica,43,3, 413438. Asmall collection of ?Aptianor ?Albian amphilestid('triconodont') mammals consisting of incomplete dentaries and maxillae with teeth, from the Khoboor localiĘ Guchin Us counĘ in Mongolia, is described. Grchinodon Troftmov' 1978 is regarded a junior subjective synonym of GobiconodonTroftmov, 1978. Heavier wear of the molariforms M3 andM4than of themore anteriorone-M2 in Gobiconodonborissiaki gives indirect evidence formolariformreplacement in this taxon. The interlocking mechanismbetween lower molariforms n Gobiconodon is of the pattern seen in Kuchneotherium and Ttnodon. The ińterlocking mechanism and the type of occlusion ally Amphilestidae with Kuehneotheriidae, from which they differ in having lower molariforms with main cusps aligned and the dentary-squamosal jaw joint (double jaw joint in Kuehneotheńdae). The main cusps in upper molariforms M3-M5 of Gobiconodon, however, show incipient tńangular arrangement. The paper gives some support to Mills' idea on the therian affinities of the Amphilestidae, although it cannot be excluded that the characters that unite the two groups developed in parallel. Because of scanty material and arnbiguĘ we assign the Amphilestidae to order incertae sedis. Key words : Mammali4 .triconodonts', Amphilestidae, Kuehneotheriidae, Early Cretaceous, Mongolia. Zofia Kiel,an-Jaworowska [zkielnn@twarda,pan.pl], InsĘtut Paleobiologii PAN, ul. Twarda 5 I /5 5, PL-00-8 I 8 Warszawa, Poland. DemberĘin Dash7eveg, Geological Institute, Mongolian Academy of Sciences, Ulan Bator, Mongolia. Introduction Beliajeva et al. (1974) reportedthe discovery of Early Cretaceous mammals at the Khoboor locality (referred to also sometimes as Khovboor), in the Guchin Us Soinon (County), Gobi Desert, Mongolia. -
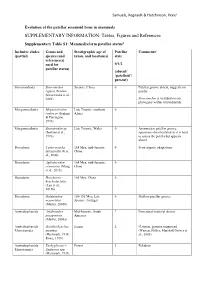
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

FROM the EARLY CRETACEOUS (BARREMIAN) WESSEX FORMATION OFTHEISLEOFWIGHT,SOUTHERNBRITAIN by STEVEN C
[Palaeontology, Vol. 49, Part 4, 2006, pp. 889–897] A GOBICONODONTID (MAMMALIA, EUTRICONODONTA) FROM THE EARLY CRETACEOUS (BARREMIAN) WESSEX FORMATION OFTHEISLEOFWIGHT,SOUTHERNBRITAIN by STEVEN C. SWEETMAN School of Earth and Environmental Sciences, University of Portsmouth, Burnaby Building, Burnaby Road, Portsmouth PO1 3QL, UK; e-mail: [email protected] Typescript received 16 June 2004; accepted in revised form 20 June 2005 Abstract: Bulk screening of Early Cretaceous (Barremian) gobiconodontid mammal in Early Cretaceous deposits of strata of the Wessex Formation, exposed in sections on Britain sheds further light on the palaeogeographical distri- the south-west and south-east coasts of the Isle of Wight, bution of an apparently successful clade of Early Creta- southern England, has resulted in the recovery of mammal ceous mammals and together with the occurrence of a remains, the first to be obtained from Wealden Group gobiconodontid in the earliest Cretaceous of North Africa strata since the early 1970s. The fauna comprises at least calls into question recent hypotheses concerning the area six taxa represented by isolated teeth and in addition, in of origin of the Gobiconodontidae and mechanisms of dis- the case of an as yet undescribed spalacotheriid, a partial persal therefrom. dentary. One of the teeth, a distal premolar, is of unique tricuspid, single-rooted morphology and represents the first Key words: Britain, Cretaceous, Gobiconodontidae, Mam- British record of the Gobiconodontidae. Discovery of a malia, palaeogeography, premolar. The Early Cretaceous (Barremian, Wealden Group) which may or may not be of mammalian origin (pers. Wessex Formation of the Isle of Wight, southern England obs. -

Two New Species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin Formations, Northeastern China
Historical Biology An International Journal of Paleobiology ISSN: 0891-2963 (Print) 1029-2381 (Online) Journal homepage: http://www.tandfonline.com/loi/ghbi20 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin To cite this article: Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin (2016) Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China, Historical Biology, 28:1-2, 14-26 To link to this article: http://dx.doi.org/10.1080/08912963.2014.977881 Published online: 01 Oct 2015. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ghbi20 Download by: [University of Sussex Library] Date: 01 October 2015, At: 18:24 Historical Biology, 2016 Vol. 28, Nos. 1–2, 14–26, http://dx.doi.org/10.1080/08912963.2014.977881 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashia*, Yuan-Qing Wangb, Chuan-Kui Lib and Xun Jinb aDepartment of Earth’s Evolution and Environment, Graduate School of Science and Engineering, Ehime University, Ehime 790-8577, Japan; bKey Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, P.R. China (Received 29 July 2014; accepted 14 October 2014) Two new gobiconodontid mammals, Gobiconodon tomidai sp.