A New Cytotype of Jacobaea Vulgaris (Asteraceae): Frequency, Morphology and Origin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Senecio Jacobaea in Northern California, an Enduring Success
ENTOMOPHAGA 35 (I), 1990, 71-77 BIOLOGICAL CONTROL OF SENECIO JACOBAEA IN NORTHERN CALIFORNIA, AN ENDURING SUCCESS R. W. PEMBERTON (t) & C. E. TURNER Rangeland Insect Lab, USDA-ARS, Montana State University, Bozeman, Montana 59717 Biological Control of Weeds Western Regional Research Center USDA-ARS, Albany, California 94710 Seneciojacobaea, a poisonous weed from Eurasia, was brought under successful biological control in the Ft. Bragg, California area by 1976, through the combined action of the defoliating cinnabar moth (Tyriajacobaeae) and a root feeding flea beetle (Longitarsusjacobaeae). In 1987, 4 previously infested Ft. Bragg sites (3 sites where control had been documented and another unstudied site) were examined. Senecio jacobaea densities at these sites were 0.0, 0.0, 0.01 and 0.18 plants/m 2, indicating both continued and improved control of the weed. The flea beetle and the cinnabar moth both persist at the sites, despite very low numbers of S.jacobaea plants. The control of S. jacobaea has resulted in the return of near natural vegetation at the 2 coastal prairie sites and regained productivity at the 2 pasture sites. KEY-WORDS : Biological control, cinnabar moth, Longitarsusjacobaeae, poiso- nous plant, Seneciojacobaea, tansy ragwort, Tyria jacobaeae. INTRODUCTION THE WEED PROBLEM Tansy ragwort, (Senecio jacobaea L. : Asteraceae) is a biennial or short lived perennial herb that is native to Europe eastward to Siberia (Harper & Wood, 1957). The plant is an introduced weed in New Zealand, Australia, South Africa, Argentina, Brazil, Canada, and the United States (Holm et al., 1979). Tansy ragwort contains toxic pyrrolizidine alkaloids which cause liver damage and death to cattle and horses that ingest it (Kingsbury, 1964 ; Harris et aL, 1984). -

Yorkhill Green Spaces Wildlife Species List
Yorkhill Green Spaces Wildlife Species List April 2021 update Yorkhill Green Spaces Species list Draft list of animals, plants, fungi, mosses and lichens recorded from Yorkhill, Glasgow. Main sites: Yorkhill Park, Overnewton Park and Kelvinhaugh Park (AKA Cherry Park). Other recorded sites: bank of River Kelvin at Bunhouse Rd/ Old Dumbarton Rd, Clyde Expressway path, casual records from streets and gardens in Yorkhill. Species total: 711 Vertebrates: Amhibians:1, Birds: 57, Fish: 7, Mammals (wild): 15 Invertebrates: Amphipods: 1, Ants: 3, Bees: 26, Beetles: 21, Butterflies: 11, Caddisflies: 2, Centipedes: 3, Earthworms: 2, Earwig: 1, Flatworms: 1, Flies: 61, Grasshoppers: 1, Harvestmen: 2, Lacewings: 2, Mayflies: 2, Mites: 4, Millipedes: 3, Moths: 149, True bugs: 13, Slugs & snails: 21, Spiders: 14, Springtails: 2, Wasps: 13, Woodlice: 5 Plants: Flowering plants: 174, Ferns: 5, Grasses: 13, Horsetail: 1, Liverworts: 7, Mosses:17, Trees: 19 Fungi and lichens: Fungi: 24, Lichens: 10 Conservation Status: NameSBL - Scottish Biodiversity List Priority Species Birds of Conservation Concern - Red List, Amber List Last Common name Species Taxon Record Common toad Bufo bufo amphiban 2012 Australian landhopper Arcitalitrus dorrieni amphipod 2021 Black garden ant Lasius niger ant 2020 Red ant Myrmica rubra ant 2021 Red ant Myrmica ruginodis ant 2014 Buff-tailed bumblebee Bombus terrestris bee 2021 Garden bumblebee Bombus hortorum bee 2020 Tree bumblebee Bombus hypnorum bee 2021 Heath bumblebee Bombus jonellus bee 2020 Red-tailed bumblebee Bombus -

Asteraceae, Senecioneae), with Nomenclatural Notes on This Name
Phytotaxa 234 (3): 271–279 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.234.3.8 Molecular and cytogenetic confirmation of the hybrid origin of Jacobaea ×mirabilis (Asteraceae, Senecioneae), with nomenclatural notes on this name GEMMA MAS DE XAXARS1, ALAIN FRIDLENDER2, TERESA GARNATJE3 & JOAN VALLÈS1 1 Laboratori de Botànica - Unitat Associada CSIC, Facultat de Farmàcia, Universitat de Barcelona. Avinguda Joan XXIII s/n, 08028 Barcelona (Catalonia, Spain); [email protected], [email protected] 2 Faculté des Sciences, Université de Provence - AMU, case 75, 3 place Victor Hugo, 13331 Marseille Cedex 3 (France); [email protected] 3 Institut Botànic de Barcelona (IBB-CSIC-ICUB), Passeig del Migdia s/n, 08038 Barcelona (Catalonia, Spain); [email protected] Abstract Jacobaea ×mirabilis is a hybrid taxon, formerly included in the genus Senecio, originating from J. adonidifolia and J. leu- cophylla as progenitors. It inhabits a few French mountain locations in the Pyrenees and in the Massif Central. In this paper, we characterize the hybrid and its parental taxa using molecular phylogenetic and cytogenetic (genome size) methods. These data are useful to confirm the hybrid status of the studied taxon. Additionally, we clarify nomenclatural issues connected with this species and fulfil the conditions for valid publication of its name in Jacobaea. The name of this hybrid is also lectotypified. Key words: genome size, homoploid hybridization, nomenclatural combination, phylogeny, Senecio ×mirabilis, typification Introduction Hybridization has classically been and is still recognized as a powerful evolutionary mechanism in plants, particularly in diversification and speciation through genetic exchange (Anderson 1949; Stebbins 1959; Arnold 2006). -
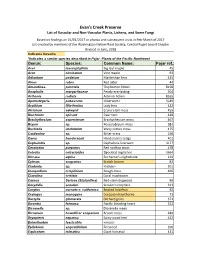
Evans-Creek-Plant-List.Pdf
Evan's Creek Preserve List of Vascular and Non‐Vascular Plants, Lichens, and Some Fungi Based on findings on 01/01/2017 or photos and subsequent visits in Feb‐March of 2017 List created by members of the Washington Native Plant Society, Central Puget Sound Chapter Revised in June, 2018 Indicates invasive *Indicates a similar species described in Pojar -Plants of the Pacific Northwest Genus: Species: Common Name: Pojar ref.: Acer macrophyllum Big leaf maple 45 Acer circinatum Vine maple 93 Adiantum pedatum Maidenhair fern 425 Alnus rubra Red alder 44 Amandinea punctata Tiny button lichen B158 Anaphalis margaritaceae Pearly everlasting 304 Arthonia radiata Asterisk lichen B165 Apometzgeria pubescens <liverwort> SL49 Arythium filix-femina Lady fern 422 Atrichum selwynii Crane's‐bill moss 455 Blechnum spicant Deer fern 420 Brachythecium asperrimum Brachythecium moss 467 Bryum sp. Rossulobryum moss S82 Buckiella undulatum Wavy cotton moss 475 Cardamine sp. Bitter‐cress 146 Carex hendersonii Henderson's sedge 401 Cephalozia sp. Cephalozia liverwort SL77 Ceratodon purpurus Red rooftop moss 478 Cetrelia cetrarioides Speckled rag lichen M64 Circaea alpina Enchanter's nightshade 210 Cytisus scoparius Scotch broom 83 Cladonia sp. <lichen> 501 Claopodium crispifoium Rough moss 466 Clavulina cristata Coral mushroom Cornus Sericea (Stolonifera) Red‐stem dogwood 90 Corydalis scouleri Scouler's corydalis 313 Corylus cornuta v. californica Beaked hazelnut 92 Crategus monogyna European hawthorne 73 Dactylis glomerata Orchard grass 371 Dicentra formosa -

(Linaria Vulgaris) and Dalmatian Toadflax (Linaria
DISSERTATION VIABILITY AND INVASIVE POTENTIAL OF HYBRIDS BETWEEN YELLOW TOADFLAX (LINARIA VULGARIS) AND DALMATIAN TOADFLAX (LINARIA DALMATICA) Submitted by Marie F.S. Turner Department of Soil and Crop Sciences In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Fall 2012 Doctoral Committee: Advisor: Sarah Ward Christopher Richards David Steingraeber George Beck Sharlene Sing Copyright by Marie Frances Sundem Turner 2012 All Rights Reserved ABSTRACT VIABILITY AND INVASIVE POTENTIAL OF HYBRIDS BETWEEN YELLOW TOADFLAX (LINARIA VULGARIS) AND DALMATIAN TOADFLAX (LINARIA DALMATICA) Although outcomes of hybridization are highly variable, it is now considered to play an important role in evolution, speciation, and invasion. Hybridization has recently been confirmed between populations of yellow (or common) toadflax (Linaria vulgaris) and Dalmatian toadflax (Linaria dalmatica) in the Rocky Mountain region of the United States. The presence of hybrid toadflax populations on public lands is of concern, as both parents are aggressive invaders already listed as noxious weeds in multiple western states. A common garden experiment was designed to measure differences in quantitative (shoot length, biomass, flowering stems, seed capsule production) phenological (time of emergence, first flowering and seed maturity) and ecophysiological (photosynthesis, transpiration and water use efficiency (WUE)) traits for yellow and Dalmatian toadflax, F1 and BC1 hybrids, as well as natural field-collected hybrids from two sites. Genotypes were cloned to produce true replicates and the entire common garden was also replicated at two locations (Colorado and Montana); physiological data were collected only in Colorado. All genotypes grew larger and were more reproductively active in Colorado than in Montana, and hybrids outperformed parent taxa across vegetative and reproductive traits indicating heterosis. -

Species List
1 of 26 Garscube 14/04/2021 species list Group Taxon Common Name Earliest Latest Records amphibian Bufo bufo Common Toad 2020 2020 1 amphibian Rana temporaria Common Frog 2005 2020 63 annelid Lumbricus 2020 2020 1 bird Accipiter nisus Sparrowhawk 2001 2021 6 bird Aegithalos caudatus Long-tailed Tit 1978 2021 13 bird Alca torda Razorbill 2019 2019 1 bird Alcedo atthis Kingfisher 1975 2020 9 bird Alle alle Little Auk 1894 1896 1 bird Anas platyrhynchos Mallard 2001 2020 4 bird Apus apus Swift 2005 2013 6 bird Ardea cinerea Grey Heron 2019 2019 1 bird Bombycilla garrulus Waxwing 2010 2010 1 bird Buteo buteo Buzzard 2007 2020 7 bird Carduelis carduelis Goldfinch 2020 2020 2 bird Certhia familiaris Treecreeper 2003 2021 10 bird Chloris chloris Greenfinch 1982 2021 7 bird Cinclus cinclus Dipper 2020 2020 1 bird Coloeus monedula Jackdaw 2020 2020 1 bird Columba oenas Stock Dove 2019 2019 3 bird Columba palumbus Woodpigeon 1962 2020 8 bird Corvus corax Raven 2019 2019 1 bird Corvus corone Carrion Crow 2010 2020 2 bird Cyanistes caeruleus Blue Tit 1982 2021 13 bird Delichon urbicum House Martin 2001 2019 4 bird Dendrocopos major Great Spotted Woodpecker 1982 2021 7 bird Emberiza citrinella Yellowhammer 1996 1996 1 bird Emberiza schoeniclus Reed Bunting 2019 2019 2 bird Erithacus rubecula Robin 2001 2021 22 bird Falco peregrinus Peregrine 1975 1982 2 bird Falco subbuteo Hobby 2017 2017 1 bird Falco tinnunculus Kestrel 2008 2008 1 bird Fringilla coelebs Chaffinch 2001 2021 6 bird Fringilla montifringilla Brambling 1994 1994 1 bird Gallinago gallinago -

Identification and Functional Characterization of the First Two
Identification and functional characterization of the first two aromatic prenyltransferases implicated in the biosynthesis of furanocoumarins and prenylated coumarins in two plant families: Rutaceae and Apiaceae Fazeelat Karamat To cite this version: Fazeelat Karamat. Identification and functional characterization of the first two aromatic prenyl- transferases implicated in the biosynthesis of furanocoumarins and prenylated coumarins in two plant families: Rutaceae and Apiaceae. Agronomy. Université de Lorraine, 2013. English. NNT : 2013LORR0029. tel-01749560 HAL Id: tel-01749560 https://hal.univ-lorraine.fr/tel-01749560 Submitted on 29 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, -

Asteraceae) from Turkey: Senecio Inops Boiss
Turk J Bot 33 (2009) 285-289 © TÜBİTAK Research Article doi:10.3906/bot-0812-3 A new taxon of Senecio (Asteraceae) from Turkey: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak Ergin HAMZAOĞLU1,*, Ümit BUDAK1, Ahmet AKSOY2 1Bozok University, Faculty of Science and Arts, Department of Biology, 66200 Yozgat - TURKEY 2Erciyes University, Faculty of Science and Arts, Department of Biology, 38039 Kayseri - TURKEY Received: 17.12.2008 Accepted: 02.06.2009 Abstract: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak (Asteraceae, Senecioneae) is described as a new subspecies from Karaman Province (Inner/South Anatolia). A Latin diagnosis, a taxonomic description, an illustration of the new subspecies, geographical distribution, and some comments on its affinity withSenecio inops Boiss. & Balansa subsp. inops are given. Key words: Asteraceae, Karaman, Senecio, taxonomy, Turkey Türkiye’den Senecio’nun (Asteraceae) yeni bir taksonu: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak Özet: Senecio inops Boiss. & Balansa subsp. karamanicus Hamzaoğlu & Budak (Asteraceae, Senecioneae) Karaman ilinden yeni bir alttür olarak tanımlandı (İç/Güney Anadolu). Yeni alttürün Latince kısa ayrımı, taksonomik betimlemesi, resmi, coğrafik yayılışı ve Senecio inops Boiss. & Balansa subsp. inops ile yakınlığı hakkında bazı yorumlar verildi. Anahtar sözcükler: Asteraceae, Karaman, Senecio, taksonomi, Türkiye Introduction infrageneric classification of Senecio difficult, and Senecioneae is one of the largest tribes of therefore, the evolutionary history of this genus is still Asteraceae, comprising about 150 genera and 3000 poorly known (Jeffrey et al., 1977; Bremer, 1994; species. Senecio L. is one of about 50 plant genera that Vincent, 1996; Mabberley, 1997). contain over 500 species. The extent of the genus The generic and infrageneric concepts of Senecio (about 1500 species) has made attempts at s.l. -

State Weed List
Class A Weeds: Non-native species whose distribution ricefield bulrush Schoenoplectus hoary alyssum Berteroa incana in Washington is still limited. Preventing new infestations and mucronatus houndstongue Cynoglossum officinale eradicating existing infestations are the highest priority. sage, clary Salvia sclarea indigobush Amorpha fruticosa Eradication of all Class A plants is required by law. sage, Mediterranean Salvia aethiopis knapweed, black Centaurea nigra silverleaf nightshade Solanum elaeagnifolium knapweed, brown Centaurea jacea Class B Weeds: Non-native species presently limited to small-flowered jewelweed Impatiens parviflora portions of the State. Species are designated for required knapweed, diffuse Centaurea diffusa control in regions where they are not yet widespread. South American Limnobium laevigatum knapweed, meadow Centaurea × gerstlaueri Preventing new infestations in these areas is a high priority. spongeplant knapweed, Russian Rhaponticum repens In regions where a Class B species is already abundant, Spanish broom Spartium junceum knapweed, spotted Centaurea stoebe control is decided at the local level, with containment as the Syrian beancaper Zygophyllum fabago knotweed, Bohemian Fallopia × bohemica primary goal. Please contact your County Noxious Weed Texas blueweed Helianthus ciliaris knotweed, giant Fallopia sachalinensis Control Board to learn which species are designated for thistle, Italian Carduus pycnocephalus knotweed, Himalayan Persicaria wallichii control in your area. thistle, milk Silybum marianum knotweed, -

Noxious Weed List Reducing Crop Yields Garden Helleborine Epipactis Helleborine Reducing Forage Quality Non-Native Lupines Lupinus Spp
Class A Weeds: Non-native species whose Washington distribution ■South American spongeplant Limnobium laevigatum ►knotweed, Japanese Polygonum cuspidatum common St. Johnswort Hypericum perforatum Spanish broom Spartium junceum is still limited. Prevention and eradication are the highest priorities. ►kochia Bassia scoparia ►common tansy Tanacetum vulgare Syrian beancaper Zygophyllum fabago Eradication of all Class A plants is required by law. ■►lesser celandine Ficaria verna ►common teasel Dipsacus fullonum Texas blueweed Helianthus ciliaris ►loosestrife, garden Lysimachia vulgaris curlyleaf pondweed Potamogeton crispus Class B Weeds: Non-native species presently limited to portions of thistle, Italian Carduus pycnocephalus ►loosestrife, purple Lythrum salicaria English hawthorn Crataegus monogyna the State. Species are designated for control in regions where they thistle, milk Silybum marianum ►loosestrife, wand Lythrum virgatum ►English and Irish ivy - four Hedera helix 'Baltica’, are not yet widespread. Preventing new infestations in these areas thistle, slenderflower Carduus tenuiflorus ►Malta starthistle Centaurea melitensis cultivars only 'Pittsburgh', & 'Star'; H. hibernica is a high priority. In regions where a Class B species is abundant, variable-leaf milfoil Myriophyllum heterophyllum 'Hibernica' control is decided locally and containment is the primary goal. wild four-o'clock Mirabilis nyctaginea ►parrotfeather Myriophyllum aquaticum Eurasian watermilfoil hybrid Myriophyllum spicatum x M. ►perennial pepperweed Lepidium latifolium -

Evaluating the Development and Potential Ecological Impact of Genetically Engineered Taraxacum Kok-Saghyz
Evaluating the Development and Potential Ecological Impact of Genetically Engineered Taraxacum kok-saghyz DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Brian J. Iaffaldano Graduate Program in Horticulture and Crop Science The Ohio State University 2016 Dissertation Committee: Professor Katrina Cornish, Advisor Professor John Cardina Professor David Francis Professor Allison Snow Copyrighted by Brian J. Iaffaldano 2016 Abstract Natural rubber is a biopolymer with irreplaceable properties, necessary in tires, medical devices and many other applications. Nearly all natural rubber production is dependent on a single species, Hevea brasiliensis. Hevea has several disadvantages, including a long life cycle, epidemic diseases, and rising production costs which have led to interest in developing new sources of rubber with similar quality to Hevea. One species that meets this criterion is Taraxacum kok-saghyz (TK), a widely adapted species of dandelion that can produce substantial amounts of rubber in its roots in an annual growing period. Shortcomings of TK include an inability to compete with many weeds, resulting in poor establishment and yields. In addition, there is variability in the amount of rubber produced, plant vigor, and seed establishment. In order to address these shortcomings, genetic engineering or breeding may be used to introduce herbicide resistance and allocate more resources to rubber production. We have demonstrated stable transformation in Taraxacum species using Agrobacterium rhizogenes to introduce genes of interest as well has hairy root phenotypes. Inoculated roots were subjected to selection by kanamycin and glufosinate and allowed to regenerate into plantlets without any hormonal treatments or additional manipulations. -

Occurrence of Nine Pyrrolizidine Alkaloids in Senecio Vulgaris L
plants Article Occurrence of Nine Pyrrolizidine Alkaloids in Senecio vulgaris L. Depending on Developmental Stage and Season Jens Flade 1,2,3 , Heidrun Beschow 1, Monika Wensch-Dorendorf 4, Andreas Plescher 2 and Wim Wätjen 3,* 1 Plant Nutrition, Institute of Agricultural and Nutritional Sciences, Martin-Luther-University Halle-Wittenberg, Betty-Heimann-Strasse 3, 06120 Halle/Saale, Germany; [email protected] (J.F.); [email protected] (H.B.) 2 PHARMAPLANT Arznei- und Gewürzpflanzen Forschungs- und Saatzucht GmbH, Am Westbahnhof 4, 06556 Artern, Germany; [email protected] 3 Biofunctionality of Secondary Plant Compounds, Institute of Agricultural and Nutritional Sciences, Martin-Luther-University Halle-Wittenberg, Weinbergweg 22, 06120 Halle/Saale, Germany 4 AG Biometrie und Agrarinformatik, Institute of Agricultural and Nutritional Sciences, Martin-Luther-University Halle-Wittenberg, Karl-Freiherr-von-Fritsch-Strasse 4, 06120 Halle/Saale, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-345-5522380 Received: 16 January 2019; Accepted: 19 February 2019; Published: 5 March 2019 Abstract: The contamination of phytopharmaceuticals and herbal teas with toxic plants is an increasing problem. Senecio vulgaris L. is a particularly noxious weed in agricultural and horticultural crops due to its content of toxic pyrrolizidine alkaloids (PAs). Since some of these compounds are carcinogenic, the distribution of this plant should be monitored. The amount of PAs in S. vulgaris is affected by various factors. Therefore, we investigated the occurrence of PAs depending on the developmental stage and season. A systematic study using field-plot experiments (four seasons, five developmental stages of the plants: S1 to S5) was performed and the PA concentration was determined via LC-MS/MS analysis.