E. E. E.M., Contains Some Hypophosphorous Acid, Is Used To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -
![United States Patent [19] [11] Patent Number: 5,368,832 Buckholtz Et A1](https://docslib.b-cdn.net/cover/1511/united-states-patent-19-11-patent-number-5-368-832-buckholtz-et-a1-641511.webp)
United States Patent [19] [11] Patent Number: 5,368,832 Buckholtz Et A1
USOO5368832A United States Patent [19] [11] Patent Number: 5,368,832 Buckholtz et a1. [45] Date of Patent: Nov. 29, 1994 [54] ZERO DISCI'IARGE PRQCESS FOR 4,330,515 5/1982 Campbell .......................... .. 423/316 MANUFACI'URING OF PHOSPHOROUS 4,380,531 4/1983 Wisnouskas ....................... .. 423/316 ACID AND HYPOPHOSPHOROUS ACID FOREIGN PATENT DOCUMENTS [75] 1I1v¢m°rs= HWY E- Buckh‘?tz, Lewiswn; 0007493 2/1980 European Pat. Off. .......... .. 423/317 Mohan S. Saran, Grand Island, both 254166 11/1986 Japan _ Of N.Y.; Frederick C. Leiterf, 7900920 11/1979 WIPO ............................... .. 423/316 Madison; David A. Flautt, Ashtabula, both of Ohio Primary iExaminer-Michael Lewis Assistant Examiner—-Stephen G. Kalinchak [73] Assignee: gggiggg?gg? Corporation’ gltltglrgey, Agent, or Firm-Wayne A. Jones; Richard D. [21] Appl. No.: 711,841 [57] ' ABS Cr [22] Flled' : Jun . 7 ’ 1991 Dlsclosed. 1s. a method of making. phosphorous acid. or [51] Int. Cl.5 .................. .. C01B 25/165; COlB 25/163 hypophosphomus acid by reacting hydrogen chloride U:S. C1. ................................... .. 423/ 316; 423/ 317 with a sodium phosphite or a sodium hypophosphite’ [58] Field of Search ............. .. 423/ 167, 307, 316, 317, respectively, in the presence of water to precipitate 423/321 R sodium chloride crystals and form the acid. The acid is [56] References Cited separated from the sodium chloride crystals and-can be US PATENT DOCUMENTS passed through amon exchange column that 1s pref erably loaded with phosphite or hypophosphite lons, 2,595,198 4/1952 Leffore et a1. ............ .. 423/316 respectively, to remove'residual chloride ions_ 2,711,388 6/1955 Mottern et a1. -

EXTRACTION EQUILIBRIA of HYPOPHOSPHOROUS, PHOSPHOROUS and PHOSPHORIC ACIDS by TOLUENE SOLUTION of TRI-N-OCTYLAMINE
ARS SEPARATORIA AA ACTA Ars Separatoria Acta 2 (2003) 125-138 www.ars_separatoria.chem. uni.torun.pl EXTRACTION EQUILIBRIA OF HYPOPHOSPHOROUS, PHOSPHOROUS AND PHOSPHORIC ACIDS BY TOLUENE SOLUTION OF TRI-n-OCTYLAMINE Takashi SANA2, Koichiro SHIOMORI1, and Yoshinobu KAWANO1* 1 Department of Applied Chemistry, Miyazaki Univ., Miyazaki 889-2192, Japan e-mail: [email protected] 2 Yoshitama Surface Finishing Co., LTD, Nobeoka 882-0024, Japan ABSTRACT The extraction equilibria of hypophosphorous, phosphorous and phosphoric acids with tri-n-octylamine as an extractant in toluene were measured at 303K. Under these conditions, undissociated acid (A') reacts with the amine (B) to form various complexes in toluene. The complexes formed and equilibrium constants were estimated by a graphical analysis method. The complexes formed in these systems were found to be A'nB type species for hypophosphorous acid, A'nBm type species for phosphorous acid, (A'B)n type species for phosphoric acid, respectively. The experimental results could be explained by reaction models and equilibrium constants for each acid. Keywords: Hypophosphorous acid; Phosphorous acid; Phosphoric acid; Tri-n-octylamine; Extraction equilibrium INTRODUCTION Solvent extraction gives the advantage of selective separation in the recycling of useful materials [1,2]. The nonelectrolytic nickel-plating wastewater consists of nickel, some organic acids, hypophosphorous acid, phosphorous acid, and phosphoric acid. In order to design a new wastewater treatment equipment of nonelectrolytic nickel-plating, it is necessary to clarify the extraction equilibrium and kinetics for each component contained in wastewater. For that fundamental knowledge, the extraction equilibrium and kinetic for nickel with 5-dodecylsalicylaldoxime [3,4] as well as * Corresponding author 125 Sana, et.al. -

(12) Patent Application Publication (10) Pub. No.: US 2008/0103330 A1 Liu Et Al
US 20080 103330A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0103330 A1 Liu et al. (43) Pub. Date: May 1, 2008 (54) PROCESS FOR THE PREPARATION OF Publication Classification HIGHLY PURIFIED, (51) Int. Cl. DALKYDTHOPHOSPHINC COMPOUNDS C07F 9/30 (2006.01) C07F 9/34 (2006.01) (76) Inventors: Leo Zhaoqing Liu, Shanghai (CN); (52) U.S. Cl. ............................................... 562/9:568/14 Gary Woodward, Northwich (57) ABSTRACT Cheshire (GB) An improved process for production of dialkyldithiophos phinic acid including Sulfurizing a purified dialkylphosphinic Correspondence Address: acid by: reacting a hypophosphorous acid or salt with a sto Kevin E. McVeigh ichiometric excess of an alpha olefin in the presence of a free RHODANC. radical initiator to form a reaction product comprising 8 Cedar Brook Drive monoalkylphosphinic acid and dialkylphosphinic acid; add Cranbury, NJ 08512-7500 ing Sufficient aqueous base to the reaction product to i) form the salts of the phosphinic acids, and ii) establish an aqueous phase and an organic phase, wherein a monoalkylphosphinic (21) Appl. No.: 11/977,620 acid solubilizes into an aqueous phase; separating the organic phase from the aqueous phase; acidifying the organic phase (22) Filed: Oct. 25, 2007 and removing the olefin from the organic phase; isolating the purified dialkylphosphinic acid product; and Sulfurizing the Related U.S. Application Data purified dialkylphosphinic acid product to form a dialky dithiophosphinic acid. The present invention also provides a (60) Provisional application No. 60/854.279, filed on Oct. process for preparing purified dialkylthiophosphinic chlo 25, 2006, provisional application No. 60/914,558, ride, and a process for preparing purified dialkylmonothio filed on Apr. -

Safe Handling and Disposal of Chemicals Used in the Illicit Manufacture of Drugs
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Safe handling and disposal of chemicals used in the illicit manufacture of drugs United Nations publication USD 26 Printed in Austria ISBN 978-92-1-148266-9 Sales No. E.11.XI.14 ST/NAR/36/Rev.1 V.11-83777—September*1183777* 2011—300 Guidelines for the Safe handling and disposal of chemicals used in the illlicit manufacture of drugs UNITED NATIONS New York, 2011 Symbols of United Nations documents are composed of letters combined with figures. Mention of such symbols indicates a reference to a United Nations document. ST/NAR/36/Rev.1 UNITED NATIONS PUBLICATION Sales No. E.11.XI.14 ISBN 978-92-1-148266-9 eISBN 978-92-1-055160-1 © United Nations, September 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Requests for permission to reproduce this work are welcomed and should be sent to the Secretary of the Publications Board, United Nations Headquarters, New York, N.Y. 10017, U.S.A. or also see the website of the Board: https://unp.un.org/Rights.aspx. Governments and their institutions may reproduce this work without prior authoriza- tion but are requested to mention the source and inform the United Nations of such reproduction. -

Process for the Production of Substituted Aromatic Hydrocarbons
Europaisches Patentamt (19) European Patent Office Office europeenpeen des brevets EP 0 663 379 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) intci.6: C07C 17/35, C07C 25/13 of the grant of the patent: 23.12.1998 Bulletin 1998/52 (21) Application number: 95300226.8 (22) Date of filing: 13.01.1995 (54) Process for the production of substituted aromatic hydrocarbons from corresponding anilines by dediazoniation Verfahren zur Herstellung von substituierten aromatischen Kohlenwasserstoffen durch Dediazonierung der ubereinstimmenden Anilinen Procede pour la production d'hydrocarbures aromatiques substitues par dediazonisation des anilines correspondantes (84) Designated Contracting States: (74) Representative: AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL Ellis-Jones, Patrick George Armine et al PT SE J.A. KEMP & CO. 14 South Square (30) Priority: 13.01.1994 GB 9400569 Gray's Inn London WC1R 5LX (GB) (43) Date of publication of application: 19.07.1995 Bulletin 1995/29 (56) References cited: WO-A-93/19026 (73) Proprietor: RHODIA LIMITED Watford, Herts WD1 1QM (GB) • CHEMICAL ABSTRACTS, vol. 115, no. 15, 14 October 1991, Columbus, Ohio, US; abstract no. (72) Inventors: 158599, K AKIN AMI T ET AL 'Indirect synthesis • Mercier, Claude of bromo-substituted aromatic compounds' & Sneyd Park, Bristol BS9 1 RU (GB) UBE KOGYO KOTO SENMON GAKKO KENKYU • Scott, Graham Vaughan HOKOKU (UKKHDQ,03864359);91; VOL.37,; Chew-Magna, Avon BS18 8SX (GB) PP.43-7, UBE TECH. COLL.;UBE; JAPAN (JP) • PATENT ABSTRACTS OF JAPAN vol. 015, no. 163 (C-0826) 24 April 1991 & JP-A-03 034 944 (TOOKEMU PROD:KK) 14 February 1991 DO O) Is- CO CO CO CO Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice the Patent Office of the Notice of shall be filed in o to European opposition to European patent granted. -

21 CFR Ch. I (4–1–12 Edition) § 184.1764
§ 184.1764 21 CFR Ch. I (4–1–12 Edition) limitation other than current good § 184.1768 Sodium lactate. manufacturing practice. The affirma- (a) Sodium lactate (C3H5O3Na, CAS tion of this ingredient as generally rec- Reg. No. 72–17–3) is the sodium salt of ognized as safe (GRAS) as a direct lactic acid. It is prepared commercially human food ingredient is based upon by the neutralization of lactic acid the following current good manufac- with sodium hydroxide. turing practice conditions of use: (b) The ingredient must be of a pu- (1) The ingredient is used as a pH rity suitable for its intended use. control agent as defined in § 170.3(o)(23) (c) In accordance with § 184.1(b)(1), of this chapter and as a processing aid the ingredient is used in food with no as defined in § 170.3(o)(24) of this chap- limitation other than current good ter. manufacturing practice. This regula- (2) The ingredient is used in foods at tion does not authorize its use in in- levels not to exceed current good man- fant foods and infant formulas. The af- ufacturing practice. firmation of this ingredient as gen- (d) Prior sanctions for this ingredient erally recognized as safe (GRAS) as a different from the uses established in direct human food ingredient is based this section do not exist or have been upon the following current good manu- waived. facturing practice conditions of use: (1) The ingredient is used as an emul- [48 FR 52444, Nov. 18, 1983] sifier as defined in § 170.3(o)(8) of this chapter; a flavor enhancer as defined in § 184.1764 Sodium hypophosphite. -

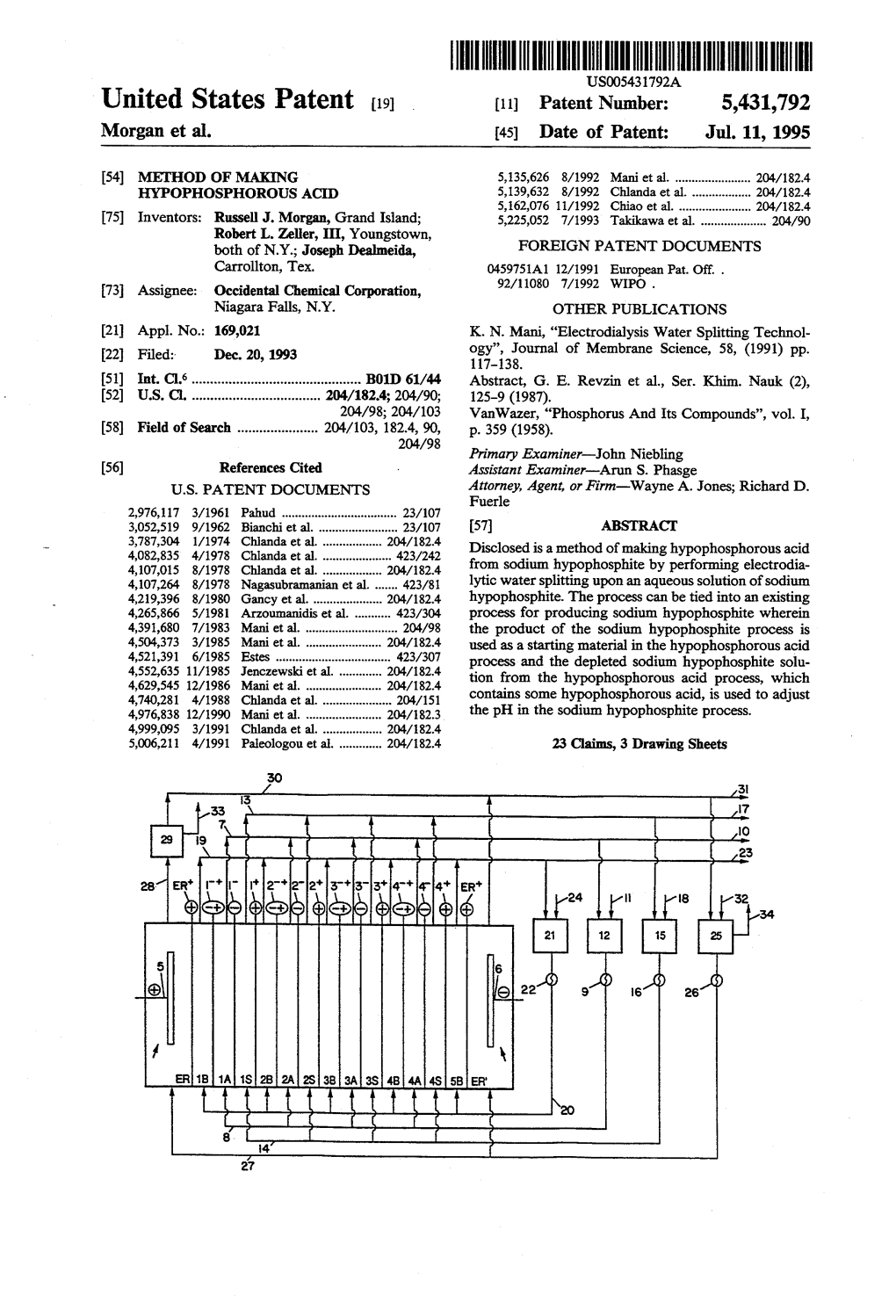
Electrolytic Production of Hypophosphorous Acid
Patentamt Europaisches || || 1 1| || || || || || || 1 1|| || || (19) J European Patent Office Office europeen des brevets (11) EP 0 701 860 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) |nt. CI.6: B01 D 61/44, C01 B 25/1 65, 20.03.1996 Bulletin 1996/12 C25B 1/22 (21) Application number: 95111697.9 (22) Date of filing: 25.07.1995 (84) Designated Contracting States: • Thomson, Donald CH DE FR GB IT LI NL Northport, New York 1 1 768 (US) • Garay, Luis Henry (30) Priority: 16.09.1994 US 307923 Rockville Center, New York 1 1 570 (US) (71 ) Applicant: LeaRonal, Inc. (74) Representative: Hansen, Bernd, Dr. Dipl.-Chem. et Freeport, N.Y. 11 520 (US) al Hoffmann, Eitle & Partner, (72) Inventors: Patentanwalte, • Nobel, Fred I. Arabellastrasse 4 Sands Point, New York 1 1 050 (US) D.81 925 Munchen (DE) • Brasch, William Nesconset, New York 1 1 767 (US) (54) Electrolytic production of hypophosphorous acid (57) Methods for preparing hypophosphorous acid the insoluble anode to a cathode (24, 124) in electrical are disclosed comprising contacting an insoluble anode contact with the aqueous solution to generate H+ ions in (12,112) with an aqueous solution of hypophosphite ani- the aqueous solution thereby forming a hypophospho- ons (27, 127) and applying a current (30, 130) through rous acid solution. FIG. 1 o CO CO o r»- o Q_ LU Printed by Rank Xerox (UK) Business Services 2.9.13/3.4 1 EP 0 701 86050 A1 2 Description the anions are transported across an anion permeable membrane into the anolyte, where they are converted to FIELD OF THE INVENTION acids or halogens. -

5439 Hypophosphorous Acid
Material Safety Data Sheet 255 Norman. EMERGENCY NUMBERS: Lachine (Montreal), Que (USA) CHEMTREC : 1(800) 424-9300 (24hrs) H8R 1A3 (CAN) CANUTEC : 1(613) 996-6666 (24hrs) (USA) Anachemia : 1(518) 297-4444 (CAN) Anachemia : 1(514) 489-5711 WHMIS Protective Clothing TDG Road/Rail WHMIS CLASS: E TDG CLASS: 8 PIN: UN1760 PG: III Section I. Product Identification and Uses Product name HYPOPHOSPHOROUS ACID CI# Not available. Chemical formula H3PO2 CAS# 6303-21-5 Synonyms Potassium citrate monohydrate, Tripotassium Code AC-5110P 2-hydroxy-1,2,3-propanecarboxylic acid, Tripotassium citrate monohydrate, AC-5110P, 47702 Formula weight 66.00 Supplier Anachemia Canada. Supersedes 255 Norman. Lachine (Montreal), Que H8R 1A3 Material uses For laboratory use only. Section II. Ingredients Name CAS # % TLV 1) HYPOPHOSPHOROUS ACID 6303-21-5 49-52 Not established by ACGIH 2) SODIUM SULFATE 7757-82-6 0-0.4 Not established by ACGIH 3) WATER 7732-18-5 48-51 Not established by ACGIH Toxicity values of the HYPOPHOSPHOROUS ACID: hazardous ingredients LD50: Not available. LC50: Not available. Section III. Physical Data HYPOPHOSPHOROUS ACID page 2/4 Physical state and Clear, colorless liquid. appearance / Odor pH (1% soln/water) Not available. Odor threshold Not available. Percent volatile 100% (w/w). ( Water). Freezing point -25°C Boiling point 108°C Specific gravity 1.21- 1.23 @ 25°C (Water = 1) Vapor density Not applicable. Vapor pressure Not applicable. Water/oil dist. coeff. Not available. Evaporation rate Not available. Solubility Miscible in water. Section IV. Fire and Explosion Data Flash point Not applicable. Flammable limits Not applicable. -

The Effect of Monovalent Cations on Electroless Nickel Plating
The Effect of Monovalent Cations On Electroless Nickel Plating By Glenn O. Mallory and Konrad Parker + + + The effect of increasing concentrations of NH4 , Li , Na of the Ni-P deposit deteriorate, as evidenced by increasing and K+ on plating rate and bath stability were investi- porosity and tensile stress. Blistering on aluminum is com- gated in a lactate-acetate EN plating solution. The mon after three MTOs. relationship between sulfate and phosphite salt The solubilities of alkali phosphite and sulfate salts are solubilities and metal turnovers was studied. Deposi- shown in Tables 2 and 3. As expected, the ammonium salts tion potentials, microstructures and phosphorus are most soluble. The nickel and lithium phosphites have the contents were also determined. lowest solubilities in water. Because a mixture of sodium and potassium sulfates is present in the used plating bath, the ince its industrial development in the 1950s, electro- actual phosphite solubilities are lower than in water alone. less nickel (EN) has found increasing use on a wide The nickel phosphite solubility depends on the chelating variety of parts, primarily for functional engineering acids used in the EN formulation, and which control the “free S applications requiring corrosion and/or wear resis- nickel” ion concentration.2 tance. Although its growth has slowed in the last few years, Sodium and potassium sulfate often crystallize when the the total value of EN coatings in the U.S. is estimated at $200 spent bath is stored below 5 °C. The phosphite anion can be million.1 precipitated as the calcium or magnesium salt; however, the EN plating has three major disadvantages compared to plating solution will then contain calcium or magnesium electrolytic nickel plating: cations that impair deposit properties.3 The use of lithium • High chemical cost ($0.40 to 0.70/L) salts in an EN bath has not been previously reported. -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 27-May-2008 Revision Date 27-Dec-2020 Revision Number 2 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Hypophosphorous acid, 50% w/w aqueous solution Cat No. : 14142 Synonyms Phosphinic acid. Molecular Formula H3 O2 P Unique Formula Identifier (UFI) YG7D-UUM6-1W01-DTWD 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) Poison Centre - Emergency Ireland : National Poisons Information Centre (NPIC) - information services 01 809 2166 (8am-10pm, 7 days a week) Malta : +356 2395 2000 Cyprus : +357 2240 5611 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards ______________________________________________________________________________________________ ALFAA14142 Page 1 / 10 SAFETY DATA SHEET Hypophosphorous acid, 50% w/w aqueous solution Revision Date 27-Dec-2020 ______________________________________________________________________________________________ Based on available data, the classification criteria are not met Health hazards Skin Corrosion/Irritation Category 1 B (H314) Serious Eye Damage/Eye Irritation Category 1 (H318) Environmental hazards Based on available data, the classification criteria are not met Full text of Hazard Statements: see section 16 2.2.