Radioactivity and Nuclear Physics 1113
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Computing ATOMIC NUCLEI
UNIVERSAL NUCLEAR ENERGY DENSITY FUNCTIONAL Computing ATOMIC NUCLEI Petascale computing helps disentangle the nuclear puzzle. The goal of the Universal Nuclear Energy Density Functional (UNEDF) collaboration is to provide a comprehensive description of all nuclei and their reactions based on the most accurate knowledge of the nuclear interaction, the most reliable theoretical approaches, and the massive use of computer power. Science of Nuclei the Hamiltonian matrix. Coupled cluster (CC) Nuclei comprise 99.9% of all baryonic matter in techniques, which were formulated by nuclear sci- the Universe and are the fuel that burns in stars. entists in the 1950s, are essential techniques in The rather complex nature of the nuclear forces chemistry today and have recently been resurgent among protons and neutrons generates a broad in nuclear structure. Quantum Monte Carlo tech- range and diversity in the nuclear phenomena that niques dominate studies of phase transitions in can be observed. As shown during the last decade, spin systems and nuclei. These methods are used developing a comprehensive description of all to understand both the nuclear and electronic nuclei and their reactions requires theoretical and equations of state in condensed systems, and they experimental investigations of rare isotopes with are used to investigate the excitation spectra in unusual neutron-to-proton ratios. These nuclei nuclei, atoms, and molecules. are labeled exotic, or rare, because they are not When applied to systems with many active par- typically found on Earth. They are difficult to pro- ticles, ab initio and configuration interaction duce experimentally because they usually have methods present computational challenges as the extremely short lifetimes. -

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -
Pair Energy of Proton and Neutron in Atomic Nuclei
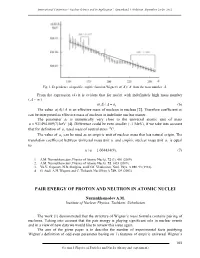
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

A Study on the Wireless Power Transfer Efficiency of Electrically Small, Perfectly Conducting Electric and Magnetic Dipoles
A study on the wireless power transfer efficiency of electrically small, perfectly conducting electric and magnetic dipoles Article Published Version Open access Moorey, C. L. and Holderbaum, W. (2017) A study on the wireless power transfer efficiency of electrically small, perfectly conducting electric and magnetic dipoles. Progress In Electromagnetics Research C, 77. pp. 111-121. ISSN 1937- 8718 doi: https://doi.org/10.2528/PIERC17062304 Available at http://centaur.reading.ac.uk/75906/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.2528/PIERC17062304 To link to this article DOI: http://dx.doi.org/10.2528/PIERC17062304 Publisher: EMW Publishing All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Progress In Electromagnetics Research C, Vol. 77, 111–121, 2017 A Study on the Wireless Power Transfer Efficiency of Electrically Small, Perfectly Conducting Electric and Magnetic Dipoles Charles L. Moorey1 and William Holderbaum2, * Abstract—This paper presents a general theoretical analysis of the Wireless Power Transfer (WPT) efficiency that exists between electrically short, Perfect Electric Conductor (PEC) electric and magnetic dipoles, with particular relevance to near-field applications. The figure of merit for the dipoles is derived in closed-form, and used to study the WPT efficiency as the criteria of interest. -

Power Waveforming: Wireless Power Transfer Beyond Time Reversal
IEEE TRANSACTIONS ON SIGNAL PROCESSING, VOL. 64, NO. 22, NOVEMBER 15, 2016 5819 Power Waveforming: Wireless Power Transfer Beyond Time Reversal Meng-Lin Ku, Member, IEEE, Yi Han, Hung-Quoc Lai, Member, IEEE, Yan Chen, Senior Member, IEEE,andK.J.RayLiu, Fellow, IEEE Abstract—This paper explores the idea of time-reversal (TR) services [1]. In particular, such an embarrassment is unavoid- technology in wireless power transfer to propose a new wireless able when wireless devices are untethered to a power grid and power transfer paradigm termed power waveforming (PW), where can only be supplied by batteries with limited capacity [2]. In a transmitter engages in delivering wireless power to an intended order to prolong the network lifetime, one immediate solution receiver by fully utilizing all the available multipaths that serve is to frequently replace batteries before the battery is exhausted, as virtual antennas. Two power transfer-oriented waveforms, en- ergy waveform and single-tone waveform, are proposed for PW but unfortunately, this strategy is inconvenient, costly and dan- power transfer systems, both of which are no longer TR in essence. gerous for some emerging wireless applications, e.g., sensor The former is designed to maximize the received power, while the networks in monitoring toxic substance. latter is a low-complexity alternative with small or even no perfor- Recently, energy harvesting has attracted a lot of attention in mance degradation. We theoretically analyze the power transfer realizing self-sustainable wireless communications with perpet- gain of the proposed power transfer system over the direct trans- ual power supplies [3], [4]. Being equipped with a rechargeable mission scheme, which can achieve about 6 dB gain, under various battery, a wireless device is solely powered by the scavenged en- channel power delay profiles and show that the PW is an ideal ergy from the natural environment such as solar, wind, motion, paradigm for wireless power transfer because of its inherent abil- vibration and radio waves. -

1 PROF. CHHANDA SAMANTA, Phd PAPERS in PEER REVIEWED INTERNATIONAL JOURNALS: 1. C. Samanta, T. A. Schmitt, “Binding, Bonding A
PROF. CHHANDA SAMANTA, PhD PAPERS IN PEER REVIEWED INTERNATIONAL JOURNALS: 1. C. Samanta, T. A. Schmitt, “Binding, bonding and charge symmetry breaking in Λ- hypernuclei”, arXiv:1710.08036v2 [nucl-th] (to be published) 2. T. A. Schmitt, C. Samanta, “A-dependence of -bond and charge symmetry energies”, EPJ Web Conf. 182, 03012 (2018) 3. Chhanda Samanta, Superheavy Nuclei to Hypernuclei: A Tribute to Walter Greiner, EPJ Web Conf. 182, 02107 (2018) 4. C. Samanta with X Qiu, L Tang, C Chen, et al., “Direct measurements of the lifetime of medium-heavy hypernuclei”, Nucl. Phys. A973, 116 (2018); arXiv:1212.1133 [nucl-ex] 5. C. Samanta with with S. Mukhopadhyay, D. Atta, K. Imam, D. N. Basu, “Static and rotating hadronic stars mixed with self-interacting fermionic Asymmetric Dark Matter”, The European Physical Journal C.77:440 (2017); arXiv:1612.07093v1 6. C. Samanta with R. Honda, M. Agnello, J. K. Ahn et al, “Missing-mass spectroscopy with 6 − + 6 the Li(π ,K )X reaction to search for ΛH”, Phys. Rev. C 96, 014005 (2017); arXiv:1703.00623v2 [nucl-ex] 7. C. Samanta with T. Gogami, C. Chen, D. Kawama et al., “Spectroscopy of the neutron-rich 7 hypernucleus He from electron scattering”, Phys. Rev. C94, 021302(R) (2016); arXiv:1606.09157 8. C. Samanta with T. Gogami, C. Chen, D. Kawama,et al., ”High Resolution Spectroscopic 10 Study of ΛBe”, Phys. Rev. C93, 034314(2016); arXiv:1511.04801v1[nucl-ex] 9. C. Samanta with L. Tang, C. Chen, T. Gogami et al., “The experiments with the High 12 Resolution Kaon Spectrometer at JLab Hall C and the new spectroscopy of ΛB hypernuclei, Phys. -

Current Status of Equation of State in Nuclear Matter and Neutron Stars
Open Issues in Understanding Core Collapse Supernovae June 22-24, 2004 Current Status of Equation of State in Nuclear Matter and Neutron Stars J.R.Stone1,2, J.C. Miller1,3 and W.G.Newton1 1 Oxford University, Oxford, UK 2Physics Division, ORNL, Oak Ridge, TN 3 SISSA, Trieste, Italy Outline 1. General properties of EOS in nuclear matter 2. Classification according to models of N-N interaction 3. Examples of EOS – sensitivity to the choice of N-N interaction 4. Consequences for supernova simulations 5. Constraints on EOS 6. High density nuclear matter (HDNM) 7. New developments Equation of State is derived from a known dependence of energy per particle of a system on particle number density: EA/(==En) or F/AF(n) I. E ( or Boltzman free energy F = E-TS for system at finite temperature) is constructed in a form of effective energy functional (Hamiltonian, Lagrangian, DFT/EFT functional or an empirical form) II. An equilibrium state of matter is found at each density n by minimization of E (n) or F (n) III. All other related quantities like e.g. pressure P, incompressibility K or entropy s are calculated as derivatives of E or F at equilibrium: 2 ∂E ()n ∂F ()n Pn()= n sn()=− | ∂n ∂T nY, p ∂∂P()nnEE() ∂2 ()n Kn()==9 18n +9n2 ∂∂nn∂n2 IV. Use as input for model simulations (Very) schematic sequence of equilibrium phases of nuclear matter as a function of density: <~2x10-4fm-3 ~2x10-4 fm-3 ~0.06 fm-3 Nuclei in Nuclei in Neutron electron gas + ‘Pasta phase’ Electron gas ~0.1 fm-3 0.3-0.5 fm-3 >0.5 fm-3 Nucleons + n,p,e,µ heavy baryons Quarks ??? -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

The Exponential Function
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln MAT Exam Expository Papers Math in the Middle Institute Partnership 5-2006 The Exponential Function Shawn A. Mousel University of Nebraska-Lincoln Follow this and additional works at: https://digitalcommons.unl.edu/mathmidexppap Part of the Science and Mathematics Education Commons Mousel, Shawn A., "The Exponential Function" (2006). MAT Exam Expository Papers. 26. https://digitalcommons.unl.edu/mathmidexppap/26 This Article is brought to you for free and open access by the Math in the Middle Institute Partnership at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in MAT Exam Expository Papers by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The Exponential Function Expository Paper Shawn A. Mousel In partial fulfillment of the requirements for the Masters of Arts in Teaching with a Specialization in the Teaching of Middle Level Mathematics in the Department of Mathematics. Jim Lewis, Advisor May 2006 Mousel – MAT Expository Paper - 1 One of the basic principles studied in mathematics is the observation of relationships between two connected quantities. A function is this connecting relationship, typically expressed in a formula that describes how one element from the domain is related to exactly one element located in the range (Lial & Miller, 1975). An exponential function is a function with the basic form f (x) = ax , where a (a fixed base that is a real, positive number) is greater than zero and not equal to 1. The exponential function is not to be confused with the polynomial functions, such as x 2. One way to recognize the difference between the two functions is by the name of the function. -

2.3 Neutrino-Less Double Electron Capture - Potential Tool to Determine the Majorana Neutrino Mass by Z.Sujkowski, S Wycech
DEPARTMENT OF NUCLEAR SPECTROSCOPY AND TECHNIQUE 39 The above conservatively large systematic hypothesis. TIle quoted uncertainties will be soon uncertainty reflects the fact that we did not finish reduced as our analysis progresses. evaluating the corrections fully in the current analysis We are simultaneously recording a large set of at the time of this writing, a situation that will soon radiative decay events for the processes t e'v y change. This result is to be compared with 1he and pi-+eN v y. The former will be used to extract previous most accurate measurement of McFarlane the ratio FA/Fv of the axial and vector form factors, a et al. (Phys. Rev. D 1984): quantity of great and longstanding interest to low BR = (1.026 ± 0.039)'1 I 0 energy effective QCD theory. Both processes are as well as with the Standard Model (SM) furthermore very sensitive to non- (V-A) admixtures in prediction (Particle Data Group - PDG 2000): the electroweak lagLangian, and thus can reveal BR = (I 038 - 1.041 )*1 0-s (90%C.L.) information on physics beyond the SM. We are currently analyzing these data and expect results soon. (1.005 - 1.008)* 1W') - excl. rad. corr. Tale 1 We see that even working result strongly confirms Current P1IBETA event sxpelilnentstatistics, compared with the the validity of the radiative corrections. Another world data set. interesting comparison is with the prediction based on Decay PIBETA World data set the most accurate evaluation of the CKM matrix n >60k 1.77k element V d based on the CVC hypothesis and ihce >60 1.77_ _ _ results -

Using Odes to Model Drug Concentrations Within the Field of Pharmacokinetics Andrea Mcnally Augustana College, Rock Island Illinois
CORE Metadata, citation and similar papers at core.ac.uk Provided by Augustana College, Illinois: Augustana Digital Commons Augustana College Augustana Digital Commons Mathematics: Student Scholarship & Creative Mathematics Works Spring 2016 Using ODEs to Model Drug Concentrations within the Field of Pharmacokinetics Andrea McNally Augustana College, Rock Island Illinois Follow this and additional works at: http://digitalcommons.augustana.edu/mathstudent Part of the Applied Mathematics Commons Augustana Digital Commons Citation McNally, Andrea. "Using ODEs to Model Drug Concentrations within the Field of Pharmacokinetics" (2016). Mathematics: Student Scholarship & Creative Works. http://digitalcommons.augustana.edu/mathstudent/2 This Student Paper is brought to you for free and open access by the Mathematics at Augustana Digital Commons. It has been accepted for inclusion in Mathematics: Student Scholarship & Creative Works by an authorized administrator of Augustana Digital Commons. For more information, please contact [email protected]. Andrea McNally MATH-478 4/4/16 In 2009, the Food and Drug Administration discovered that individuals taking over-the- counter pain medications containing acetaminophen were at risk of unintentional overdose because these patients would supplement the painkillers with other medications containing acetaminophen. This resulted in an increase in liver failures and death over the years. To combat this problem, the FDA decided to lower the dosage from 1000 mg to 650 mg every four hours, thus reducing this risk (U.S. Food and Drug Administration). The measures taken in this example demonstrate the concepts behind the field of Pharmacology. Pharmacology is known as the study of the uses, effects, and mode of action of drugs (“Definition of Pharmacology”).