Chapter 30 Quantum Physics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:0809.1003V5 [Hep-Ph] 5 Oct 2010 Htnadgaio Aslimits Mass Graviton and Photon I.Scr N Pcltv Htnms Limits Mass Photon Speculative and Secure III](https://docslib.b-cdn.net/cover/5435/arxiv-0809-1003v5-hep-ph-5-oct-2010-htnadgaio-aslimits-mass-graviton-and-photon-i-scr-n-pcltv-htnms-limits-mass-photon-speculative-and-secure-iii-385435.webp)
Arxiv:0809.1003V5 [Hep-Ph] 5 Oct 2010 Htnadgaio Aslimits Mass Graviton and Photon I.Scr N Pcltv Htnms Limits Mass Photon Speculative and Secure III
October 7, 2010 Photon and Graviton Mass Limits Alfred Scharff Goldhaber∗,† and Michael Martin Nieto† ∗C. N. Yang Institute for Theoretical Physics, SUNY Stony Brook, NY 11794-3840 USA and †Theoretical Division (MS B285), Los Alamos National Laboratory, Los Alamos, NM 87545 USA Efforts to place limits on deviations from canonical formulations of electromagnetism and gravity have probed length scales increasing dramatically over time. Historically, these studies have passed through three stages: (1) Testing the power in the inverse-square laws of Newton and Coulomb, (2) Seeking a nonzero value for the rest mass of photon or graviton, (3) Considering more degrees of freedom, allowing mass while preserving explicit gauge or general-coordinate invariance. Since our previous review the lower limit on the photon Compton wavelength has improved by four orders of magnitude, to about one astronomical unit, and rapid current progress in astronomy makes further advance likely. For gravity there have been vigorous debates about even the concept of graviton rest mass. Meanwhile there are striking observations of astronomical motions that do not fit Einstein gravity with visible sources. “Cold dark matter” (slow, invisible classical particles) fits well at large scales. “Modified Newtonian dynamics” provides the best phenomenology at galactic scales. Satisfying this phenomenology is a requirement if dark matter, perhaps as invisible classical fields, could be correct here too. “Dark energy” might be explained by a graviton-mass-like effect, with associated Compton wavelength comparable to the radius of the visible universe. We summarize significant mass limits in a table. Contents B. Einstein’s general theory of relativity and beyond? 20 C. -
Compton Effect
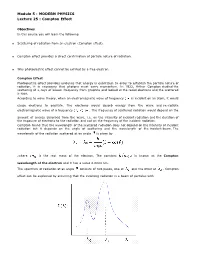
Module 5 : MODERN PHYSICS Lecture 25 : Compton Effect Objectives In this course you will learn the following Scattering of radiation from an electron (Compton effect). Compton effect provides a direct confirmation of particle nature of radiation. Why photoelectric effect cannot be exhited by a free electron. Compton Effect Photoelectric effect provides evidence that energy is quantized. In order to establish the particle nature of radiation, it is necessary that photons must carry momentum. In 1922, Arthur Compton studied the scattering of x-rays of known frequency from graphite and looked at the recoil electrons and the scattered x-rays. According to wave theory, when an electromagnetic wave of frequency is incident on an atom, it would cause electrons to oscillate. The electrons would absorb energy from the wave and re-radiate electromagnetic wave of a frequency . The frequency of scattered radiation would depend on the amount of energy absorbed from the wave, i.e. on the intensity of incident radiation and the duration of the exposure of electrons to the radiation and not on the frequency of the incident radiation. Compton found that the wavelength of the scattered radiation does not depend on the intensity of incident radiation but it depends on the angle of scattering and the wavelength of the incident beam. The wavelength of the radiation scattered at an angle is given by .where is the rest mass of the electron. The constant is known as the Compton wavelength of the electron and it has a value 0.0024 nm. The spectrum of radiation at an angle consists of two peaks, one at and the other at . -

1 the Principle of Wave–Particle Duality: an Overview
3 1 The Principle of Wave–Particle Duality: An Overview 1.1 Introduction In the year 1900, physics entered a period of deep crisis as a number of peculiar phenomena, for which no classical explanation was possible, began to appear one after the other, starting with the famous problem of blackbody radiation. By 1923, when the “dust had settled,” it became apparent that these peculiarities had a common explanation. They revealed a novel fundamental principle of nature that wascompletelyatoddswiththeframeworkofclassicalphysics:thecelebrated principle of wave–particle duality, which can be phrased as follows. The principle of wave–particle duality: All physical entities have a dual character; they are waves and particles at the same time. Everything we used to regard as being exclusively a wave has, at the same time, a corpuscular character, while everything we thought of as strictly a particle behaves also as a wave. The relations between these two classically irreconcilable points of view—particle versus wave—are , h, E = hf p = (1.1) or, equivalently, E h f = ,= . (1.2) h p In expressions (1.1) we start off with what we traditionally considered to be solely a wave—an electromagnetic (EM) wave, for example—and we associate its wave characteristics f and (frequency and wavelength) with the corpuscular charac- teristics E and p (energy and momentum) of the corresponding particle. Conversely, in expressions (1.2), we begin with what we once regarded as purely a particle—say, an electron—and we associate its corpuscular characteristics E and p with the wave characteristics f and of the corresponding wave. -

The Graviton Compton Mass As Dark Energy
Gravitation, Mathematical Physics and Field Theory Revista Mexicana de F´ısica 67 040703 1–5 JULY-AUGUST 2021 The graviton Compton mass as dark energy T. Matosa and L. L.-Parrillab aDepartamento de F´ısica, Centro de Investigacion´ y de Estudios Avanzados del IPN, Apartado Postal 14-740, 07000, CDMX, Mexico.´ bInstituto de Ciencias Nucleares, Universidad Nacional Autonoma´ de Mexico,´ Circuito Exterior C.U., Apartado Postal 70-543, 04510, CDMX, Mexico.´ Received 3 January 2021; accepted 18 February 2021 One of the greatest challenges of science is to understand the current accelerated expansion of the Universe. In this work, we show that by considering the quantum nature of the gravitational field, its wavelength can be associated with an effective Compton mass. We propose that this mass can be interpreted as dark energy, with a Compton wavelength given by the size of the observable Universe, implying that the dark energy varies depending on this size. If we do so, we find that: 1.- Even without any free constant for dark energy, the evolution of the Hubble parameter is exactly the same as for the LCDM model, so this model has the same predictions as LCDM. 2.- The density rate of the dark energy is ¤ = 0:69 which is a very similar value as the one found by the Planck satellite ¤ = 0:684. 3.- The dark energy has this value because it corresponds to the actual size of the radius of the Universe, thus the coincidence problem has a very natural explanation. 4.- It, is possible to find also a natural explanation to why observations inferred from the local distance ladder find the value H0 = 73 km/s/Mpc for the Hubble constant. -

Compton Wavelength, Bohr Radius, Balmer's Formula and G-Factors
Compton wavelength, Bohr radius, Balmer's formula and g-factors Raji Heyrovska J. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, Dolejškova 3, 182 23 Prague 8, Czech Republic. [email protected] Abstract. The Balmer formula for the spectrum of atomic hydrogen is shown to be analogous to that in Compton effect and is written in terms of the difference between the absorbed and emitted wavelengths. The g-factors come into play when the atom is subjected to disturbances (like changes in the magnetic and electric fields), and the electron and proton get displaced from their fixed positions giving rise to Zeeman effect, Stark effect, etc. The Bohr radius (aB) of the ground state of a hydrogen atom, the ionization energy (EH) and the Compton wavelengths, λC,e (= h/mec = 2πre) and λC,p (= h/mpc = 2πrp) of the electron and proton respectively, (see [1] for an introduction and literature), are related by the following equations, 2 EH = (1/2)(hc/λH) = (1/2)(e /κ)/aB (1) 2 2 (λC,e + λC,p) = α2πaB = α λH = α /2RH (2) (λC,e + λC,p) = (λout - λin)C,e + (λout - λin)C,p (3) α = vω/c = (re + rp)/aB = 2πaB/λH (4) aB = (αλH/2π) = c(re + rp)/vω = c(τe + τp) = cτB (5) where λH is the wavelength of the ionizing radiation, κ = 4πεo, εo is 2 the electrical permittivity of vacuum, h (= 2πħ = e /2εoαc) is the 2 Planck constant, ħ (= e /κvω) is the angular momentum of spin, α (= vω/c) is the fine structure constant, vω is the velocity of spin [2], re ( = ħ/mec) and rp ( = ħ/mpc) are the radii of the electron and -

Compton Shift and De Broglie Frequency
Compton shift and de Broglie frequency Raji Heyrovská J. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, Dolejskova 3, 182 23 Prague 8, Czech Republic. [email protected] Abstract. Compton scattering is usually explained in terms of the relativistic mass and momentum. Here, a mathematically equivalent and simple non-relativistic interpretation shows that the Compton frequency shift is equal to the de Broglie frequency associated with the moving charged particle (e.g., an electron). In this work, the moving electron is considered as a particle and the electromagnetic energy associated with it is shown to be proportional to the de Broglie frequency. This energy is released when its motion is arrested, as for example on a diffraction screen, where it causes the observed interfernce patterns. Thus, electrons transport electromagnetic energy from a source to a sink. Key words: Compton shift, de Broglie wavelength, wave/particle duality, transport of electromagnetic energy An introduction to Compton effect can be found in many books, e.g., see [1]. A bibliography on the various interpretations using relativistic and quantum theories can be found in [2]. Here, a simple non-relativistic view of the phenomenon is presented. Essentially, in Compton scattering, an incident X-ray of wavelength λin interacts with matter and the scattered ray has a longer wave length λθ, the value of which depends on the scattering angle θ from the incident ray. In the case of interaction with electrons, the Compton wavelength shift ∆λe,θ is given by [1], ∆λe,θ = λθ - λin = λC,e(1- cos θ) (1) where λC,e, the Compton wavelength (obtained as the Compton shift for θ = π/2) is found to be, 2 λC,e = (h/mec) [= (I/me) = 2π(e /κvω)(1/mec)] (2) h is the Planck constsnt and me is the rest mass of the electron. -

Compton Wavelength −31 8 Mce (9.109×× 10Kg )( 2.998 10M / S) =×=×=2.43 10−−3 Nm 2.43 1012 M 2.43 Pm Note: 1 Pm= 1 × 10−12 M 6 Summary: Compton Scattering
Modern Physics Unit 1: Classical Models and the Birth of Modern Physics Lecture 1.6: Compton Effect Ron Reifenberger Professor of Physics Purdue University 1 V. The Compton Effect (1923) Energy Loss Θ=30o Carbon block source of high energy photons Compton’s Apparatus (schematic) What was expected? - e f hf f hf No energy loss ! Source: Tipler and Llewellyn, Modern Physics, 4th edition2 Compton applies conservation of energy and momentum principles to explain puzzling data. Recall collision theory: Before After vi1 vi2 vf1 vf2 m m m m2 1 m22 1 Conservation of momentum: m1vf1 + m2vf2 = m1vi1 + m2vi2 Conservation of kinetic energy: 2 2 2 2 ½ m1vf1 + ½ m2vf2 = ½ m1vi1 + ½ m2vi2 3 What’s the momentum of a photon? λ Classical mechanics: p = mv p=???? E but….photon mass = 0 ⋅ from Planck E = hf ⋅ from Maxwell, the momentum of an EM Wave is: U Calculate change (see L1.03) in momentum of a p = surface when hit c by EM wave hf hf h p = = = cfλλ EM Wave h=Planck’s constant = 6.626x10-34 m2 kg/s 4 Compton’s analysis considered an X-ray photon scattering from a single electron inside a target Before After photon photon λ f electron at loses λ i rest energy me electron recoils How is λ f related to λ i ? 5 Geometry of Compton Scattering λ Initial Scattered f position of photon electron Incident photon Θ h _ λλfi−=(1 − cos Θ) mce λ i me _ Recoiling electron h 6.626×⋅ 10−34 Js ≡= Compton wavelength −31 8 mce (9.109×× 10kg )( 2.998 10m / s) =×=×=2.43 10−−3 nm 2.43 1012 m 2.43 pm Note: 1 pm= 1 × 10−12 m 6 Summary: Compton Scattering h λλfi−=(1 − cos Θ) mce energy h loss Inelastic mce λf Scattering λi λf Increases λi λi λf number of π photons h Θ= detected 2 2 mce Scattered λi = λf λi λf Beam Elastic m λ e Scattering Θ=π Θ=0 Example: EM radiation with differing wavelengths is scattered by an electron through an angle of 37o (w.r.t. -

The Particle World
The Particle World CERN Summer School Lectures 2021 Lectures 1 and 2: The Standard Model David Tong Further Reading around 240 pages! (Sorry) What are we made of? "If we consider protons and neutrons as elementary particles, we would have three kinds of elementary particles [p,n,e].... This number may seem large but, from that point of view, two is already a large number.” Paul Dirac 1933 Solvay Conference The Standard Model 12 particles + 4 forces + Higgs boson The Standard Model ContentsContentsContentsContents Contents 1 Introduction1 Introduction11 Introduction Introduction1 Introduction 1 1 1 1 1 1 Introduction1 Introduction11 Introduction Introduction1 Introduction The numbers in the table are the masses of the particles, written as multiples of the electron mass. (Hence the electron itself is assigned mass 1.) The masses of the neutrinosGravity are ElectromagnetismGravity knownGravity ElectromagnetismGravity to be very Electromagnetism Weak Electromagnetism smallGravity Strong but, Weak otherwise Electromagnetism Strong Weak Weak are Strong onlyStrong constrained Weak Strong within a window and not yet established individually. Each horizontal line of this diagram is called a generation. Hence, each generation consists of an electron-like particle, twoHiggs quarks, and a neutrino. The statement that each generation behaves the same means that, among other things, the electric charges of all electron-like particles in the first column are 1(inappropriateunits);theelectric −1 charges of all quarks in the second column are 3 and all those in the third column 2 − + 3 . All neutrinos are electrically neutral. We understand aspects of this horizontal pattern very well. In particular, various mathematical consistency conditions tell us that the particles must come in a collective of four particles, and their properties are largely fixed. -

Schrödinger Equation for an Extended Electron
Schr¨odinger equation for an extended electron Antˆonio B. Nassar Physics Department The Harvard-Westlake School 3700 Coldwater Canyon, Studio City, 91604 (USA) and Department of Sciences University of California, Los Angeles, Extension Program 10995 Le Conte Avenue, Los Angeles, CA 90024 (USA) Abstract A new quantum mechanical wave equation describing the dynamics of an extended electron is derived via Bohmian mechanics. The solution to this equation is found through a wave packet approach which establishes a direct correlation between a classical variable with a quantum variable describing the dynamics of the center of mass and the width of the electron wave packet. The approach presented in this paper gives a comparatively clearer picture than approaches using elaborative manipulation of infinite series of operators. It is shown that the new Schr¨odinger equation is free of any runaway solutions or any acausal responses. PACS: 03.65.Ta, 03.65.Sq, 03.70.+k 2 About a century ago, Lorentz [1] and Abraham [2] argued that when an electron is accelerated, there are additional forces acting due to the electron’s own electromagnetic field. However, the so-called Lorentz-Abraham equation for a point-charge electron dV 2e2 d2V m = + Fext (1) dt 3c3 dt2 was found to be unsatisfactory because, for Fext = 0, it admits runaway solutions. These solutions clearly violate the law of inertia. Since the seminal works of Lorentz and Abraham, inumerous papers and textbooks have given great consideration to the proper equation of motion of an electron.[3]-[11] The problematic runaway solutions were circumvented by Sommerfeld [5] and Page [6] by going to an extended model. -

The Nature of Λ and the Mass of the Graviton: a Critical View J.-P
The Nature of Λ and the Mass of the Graviton: a Critical View J.-P. Gazeau, M. Novello To cite this version: J.-P. Gazeau, M. Novello. The Nature of Λ and the Mass of the Graviton: a Critical View. International Journal of Modern Physics A, World Scientific Publishing, 2011, 26, pp.3697-3720. 10.1142/S0217751X11054176. hal-00105287 HAL Id: hal-00105287 https://hal.archives-ouvertes.fr/hal-00105287 Submitted on 11 Oct 2006 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The nature of Λ and the mass of the graviton: A critical view J. P. Gazeau∗ and M. Novello∗∗ ∗ Laboratoire Astroparticules et Cosmologie (APC, UMR 7164), Boite 7020 Universit´eParis 7 Denis Diderot, 2 Place Jussieu, 75251 Paris Cedex 05 Fr [email protected] ∗∗ ICRA, Centro Brasileiro de Pesquisas Fisicas, Rua Dr. Xavier Sigaud 150, CEP 22290-180, Rio de Janeiro, Brazil [email protected] October 11, 2006 Abstract The existence of a non-zero cosmological constant Λ gives rise to controversial interpretations. Is Λ a universal constant fixing the ge- ometry of an empty universe, as fundamental as the Planck constant or the speed of light in the vacuum? Its natural place is then on the left-hand side of the Einstein equation. -
![Arxiv:1704.05034V1 [Physics.Plasm-Ph] 17 Apr 2017 Lsia Potentials](https://docslib.b-cdn.net/cover/5694/arxiv-1704-05034v1-physics-plasm-ph-17-apr-2017-lsia-potentials-2665694.webp)
Arxiv:1704.05034V1 [Physics.Plasm-Ph] 17 Apr 2017 Lsia Potentials
Finding quantum effects in strong classical potentials B. Manuel Hegelich,1 Lance Labun,1 and Ou Z. Labun1, 2 1Department of Physics, University of Texas, Austin, Texas 78712 U.S.A. 2Department of Physics, University of Arizona, Tucson, Arizona 85721 U.S.A. (Dated: 14 July 2016) The long-standing challenge to describing charged particle dynamics in strong classical electromagnetic fields is how to incorporate classical radiation, classical radiation reaction and quantized photon emission into a consistent unified framework. The current, semi- classical methods to describe dynamics of quantum particles in strong classical fields also provide the theoretical framework for fundamental questions in gravity and hadron-hadron collisions, including Hawking radiation, cosmological particle production and thermalization of particles created in heavy-ion collisions. However, as we show, these methods break down for highly relativistic particles propagating in strong fields. They must therefore be im- proved and adapted for the description of laser-plasma experiments that typically involve the acceleration of electrons. Theory developed from quantum electrodynamics, together with dedicated experimental efforts, offer the best-controllable context to establish a robust, experimentally-validated foundation for the fundamental theory of quantum effects in strong classical potentials. arXiv:1704.05034v1 [physics.plasm-ph] 17 Apr 2017 2 I. INTRODUCTION: CLASSICAL AND QUANTUM RADIATION Although the photoelectric effect showed that radiation is fundamentally a quantum pro- cess, long-wavelength (λ ≫ λe = ~/mec where me is the electron mass) radiation is equally well described by solving Maxwell’s equations with a classical current as source. This classical 2 approximation works because, in weak fields, the energy of the probe ~ω = hc/λ ≪ mec is insufficient to excite the quantum structure of the electron. -

An Analytical Study on the Compton Wavelength
Transactions of the Korean Nuclear Society Autumn Meeting PyeongChang, Korea, October 30-31, 2008 An Analytical Study on the Compton Wavelength Sun-Tae Hwang and Dong-Kwan Shin TEKCIVIL Corporation 301 Songjeon B/D, 1182 Dunsan-dong, Seogu, Daejeon 302-121, Korea 1. Introduction p of a particle are related to its rest mass m 2 2 2 4 Mathematically, the Compton wavelength λc of by the invariant relation, p ∙pc - E = -m c . a particle is given by λc = h/mc, where h is the Now, by conservation of energy, Planck constant, m is the particle's rest mass 2 2 2 1/2 and c is the speed of light. For the electron as pγ +mec = p'γ +(me c +p'e ) (3) -12 a reference constant, it is about 2.426x10 m, intermediate between the size of an atomic nu- Rearranging and squaring both sides, Eq.(4) is cleus and an atom. The λc means a critical dis- obtained. tance below which certain quantum mechanical 2 2 2 2 2 2 2 effects take place for any particle. The energy pγ +p'γ -2pγp'γ+2mec(pγ-p'γ)+me c =me c +p'e of a photon of this wavelength is equal to the (4) 2 rest mass energy mec of an electron[1]. Now, Subtraction of Eq.(2) from Eq.(4) yields the λc is analytically reviewed. mec(pγ-p'γ) = pγp'γ - pγ․p'γ (5) 2. Compton Scattering Effect Finally, dividing both sides by mecpγp'γ, Eq.(6) Compton scattering effect is the result of a for the relation between the energies of the in- high energy photon colliding with an electron.