Compton Wavelength, Bohr Radius, Balmer's Formula and G-Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

Optical Properties of Bulk and Nano
Lecture 3: Optical Properties of Bulk and Nano 5 nm Course Info First H/W#1 is due Sept. 10 The Previous Lecture Origin frequency dependence of χ in real materials • Lorentz model (harmonic oscillator model) e- n(ω) n' n'' n'1= ω0 + Nucleus ω0 ω Today optical properties of materials • Insulators (Lattice absorption, color centers…) • Semiconductors (Energy bands, Urbach tail, excitons …) • Metals (Response due to bound and free electrons, plasma oscillations.. ) Optical properties of molecules, nanoparticles, and microparticles Classification Matter: Insulators, Semiconductors, Metals Bonds and bands • One atom, e.g. H. Schrödinger equation: E H+ • Two atoms: bond formation ? H+ +H Every electron contributes one state • Equilibrium distance d (after reaction) Classification Matter ~ 1 eV • Pauli principle: Only 2 electrons in the same electronic state (one spin & one spin ) Classification Matter Atoms with many electrons Empty outer orbitals Outermost electrons interact Form bands Partly filled valence orbitals Filled Energy Inner Electrons in inner shells do not interact shells Do not form bands Distance between atoms Classification Matter Insulators, semiconductors, and metals • Classification based on bandstructure Dispersion and Absorption in Insulators Electronic transitions No transitions Atomic vibrations Refractive Index Various Materials 3.4 3.0 2.0 Refractive index: n’ index: Refractive 1.0 0.1 1.0 10 λ (µm) Color Centers • Insulators with a large EGAP should not show absorption…..or ? • Ion beam irradiation or x-ray exposure result in beautiful colors! • Due to formation of color (absorption) centers….(Homework assignment) Absorption Processes in Semiconductors Absorption spectrum of a typical semiconductor E EC Phonon Photon EV ωPhonon Excitons: Electron and Hole Bound by Coulomb Analogy with H-atom • Electron orbit around a hole is similar to the electron orbit around a H-core • 1913 Niels Bohr: Electron restricted to well-defined orbits n = 1 + -13.6 eV n = 2 n = 3 -3.4 eV -1.51 eV me4 13.6 • Binding energy electron: =−=−=e EB 2 2 eV, n 1,2,3,.. -
![Arxiv:0809.1003V5 [Hep-Ph] 5 Oct 2010 Htnadgaio Aslimits Mass Graviton and Photon I.Scr N Pcltv Htnms Limits Mass Photon Speculative and Secure III](https://docslib.b-cdn.net/cover/5435/arxiv-0809-1003v5-hep-ph-5-oct-2010-htnadgaio-aslimits-mass-graviton-and-photon-i-scr-n-pcltv-htnms-limits-mass-photon-speculative-and-secure-iii-385435.webp)
Arxiv:0809.1003V5 [Hep-Ph] 5 Oct 2010 Htnadgaio Aslimits Mass Graviton and Photon I.Scr N Pcltv Htnms Limits Mass Photon Speculative and Secure III
October 7, 2010 Photon and Graviton Mass Limits Alfred Scharff Goldhaber∗,† and Michael Martin Nieto† ∗C. N. Yang Institute for Theoretical Physics, SUNY Stony Brook, NY 11794-3840 USA and †Theoretical Division (MS B285), Los Alamos National Laboratory, Los Alamos, NM 87545 USA Efforts to place limits on deviations from canonical formulations of electromagnetism and gravity have probed length scales increasing dramatically over time. Historically, these studies have passed through three stages: (1) Testing the power in the inverse-square laws of Newton and Coulomb, (2) Seeking a nonzero value for the rest mass of photon or graviton, (3) Considering more degrees of freedom, allowing mass while preserving explicit gauge or general-coordinate invariance. Since our previous review the lower limit on the photon Compton wavelength has improved by four orders of magnitude, to about one astronomical unit, and rapid current progress in astronomy makes further advance likely. For gravity there have been vigorous debates about even the concept of graviton rest mass. Meanwhile there are striking observations of astronomical motions that do not fit Einstein gravity with visible sources. “Cold dark matter” (slow, invisible classical particles) fits well at large scales. “Modified Newtonian dynamics” provides the best phenomenology at galactic scales. Satisfying this phenomenology is a requirement if dark matter, perhaps as invisible classical fields, could be correct here too. “Dark energy” might be explained by a graviton-mass-like effect, with associated Compton wavelength comparable to the radius of the visible universe. We summarize significant mass limits in a table. Contents B. Einstein’s general theory of relativity and beyond? 20 C. -

Rydberg Constant and Emission Spectra of Gases
Page 1 of 10 Rydberg constant and emission spectra of gases ONE WEIGHT RECOMMENDED READINGS 1. R. Harris. Modern Physics, 2nd Ed. (2008). Sections 4.6, 7.3, 8.9. 2. Atomic Spectra line database https://physics.nist.gov/PhysRefData/ASD/lines_form.html OBJECTIVE - Calibrating a prism spectrometer to convert the scale readings in wavelengths of the emission spectral lines. - Identifying an "unknown" gas by measuring its spectral lines wavelengths. - Calculating the Rydberg constant RH. - Finding a separation of spectral lines in the yellow doublet of the sodium lamp spectrum. INSTRUCTOR’S EXPECTATIONS In the lab report it is expected to find the following parts: - Brief overview of the Bohr’s theory of hydrogen atom and main restrictions on its application. - Description of the setup including its main parts and their functions. - Description of the experiment procedure. - Table with readings of the vernier scale of the spectrometer and corresponding wavelengths of spectral lines of hydrogen and helium. - Calibration line for the function “wavelength vs reading” with explanation of the fitting procedure and values of the parameters of the fit with their uncertainties. - Calculated Rydberg constant with its uncertainty. - Description of the procedure of identification of the unknown gas and statement about the gas. - Calculating resolution of the spectrometer with the yellow doublet of sodium spectrum. INTRODUCTION In this experiment, linear emission spectra of discharge tubes are studied. The discharge tube is an evacuated glass tube filled with a gas or a vapor. There are two conductors – anode and cathode - soldered in the ends of the tube and connected to a high-voltage power source outside the tube. -

Bohr Model of Hydrogen
Chapter 3 Bohr model of hydrogen Figure 3.1: Democritus The atomic theory of matter has a long history, in some ways all the way back to the ancient Greeks (Democritus - ca. 400 BCE - suggested that all things are composed of indivisible \atoms"). From what we can observe, atoms have certain properties and behaviors, which can be summarized as follows: Atoms are small, with diameters on the order of 0:1 nm. Atoms are stable, they do not spontaneously break apart into smaller pieces or collapse. Atoms contain negatively charged electrons, but are electrically neutral. Atoms emit and absorb electromagnetic radiation. Any successful model of atoms must be capable of describing these observed properties. 1 (a) Isaac Newton (b) Joseph von Fraunhofer (c) Gustav Robert Kirch- hoff 3.1 Atomic spectra Even though the spectral nature of light is present in a rainbow, it was not until 1666 that Isaac Newton showed that white light from the sun is com- posed of a continuum of colors (frequencies). Newton introduced the term \spectrum" to describe this phenomenon. His method to measure the spec- trum of light consisted of a small aperture to define a point source of light, a lens to collimate this into a beam of light, a glass spectrum to disperse the colors and a screen on which to observe the resulting spectrum. This is indeed quite close to a modern spectrometer! Newton's analysis was the beginning of the science of spectroscopy (the study of the frequency distri- bution of light from different sources). The first observation of the discrete nature of emission and absorption from atomic systems was made by Joseph Fraunhofer in 1814. -

Particle Nature of Matter
Solved Problems on the Particle Nature of Matter Charles Asman, Adam Monahan and Malcolm McMillan Department of Physics and Astronomy University of British Columbia, Vancouver, British Columbia, Canada Fall 1999; revised 2011 by Malcolm McMillan Given here are solutions to 5 problems on the particle nature of matter. The solutions were used as a learning-tool for students in the introductory undergraduate course Physics 200 Relativity and Quanta given by Malcolm McMillan at UBC during the 1998 and 1999 Winter Sessions. The solutions were prepared in collaboration with Charles Asman and Adam Monaham who were graduate students in the Department of Physics at the time. The problems are from Chapter 3 The Particle Nature of Matter of the course text Modern Physics by Raymond A. Serway, Clement J. Moses and Curt A. Moyer, Saunders College Publishing, 2nd ed., (1997). Coulomb's Constant and the Elementary Charge When solving numerical problems on the particle nature of matter it is useful to note that the product of Coulomb's constant k = 8:9876 × 109 m2= C2 (1) and the square of the elementary charge e = 1:6022 × 10−19 C (2) is ke2 = 1:4400 eV nm = 1:4400 keV pm = 1:4400 MeV fm (3) where eV = 1:6022 × 10−19 J (4) Breakdown of the Rutherford Scattering Formula: Radius of a Nucleus Problem 3.9, page 39 It is observed that α particles with kinetic energies of 13.9 MeV or higher, incident on copper foils, do not obey Rutherford's (sin φ/2)−4 scattering formula. • Use this observation to estimate the radius of the nucleus of a copper atom. -

Improving the Accuracy of the Numerical Values of the Estimates Some Fundamental Physical Constants
Improving the accuracy of the numerical values of the estimates some fundamental physical constants. Valery Timkov, Serg Timkov, Vladimir Zhukov, Konstantin Afanasiev To cite this version: Valery Timkov, Serg Timkov, Vladimir Zhukov, Konstantin Afanasiev. Improving the accuracy of the numerical values of the estimates some fundamental physical constants.. Digital Technologies, Odessa National Academy of Telecommunications, 2019, 25, pp.23 - 39. hal-02117148 HAL Id: hal-02117148 https://hal.archives-ouvertes.fr/hal-02117148 Submitted on 2 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Improving the accuracy of the numerical values of the estimates some fundamental physical constants. Valery F. Timkov1*, Serg V. Timkov2, Vladimir A. Zhukov2, Konstantin E. Afanasiev2 1Institute of Telecommunications and Global Geoinformation Space of the National Academy of Sciences of Ukraine, Senior Researcher, Ukraine. 2Research and Production Enterprise «TZHK», Researcher, Ukraine. *Email: [email protected] The list of designations in the text: l -
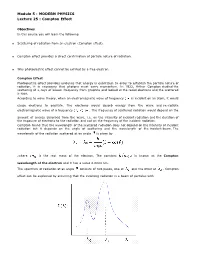
Compton Effect
Module 5 : MODERN PHYSICS Lecture 25 : Compton Effect Objectives In this course you will learn the following Scattering of radiation from an electron (Compton effect). Compton effect provides a direct confirmation of particle nature of radiation. Why photoelectric effect cannot be exhited by a free electron. Compton Effect Photoelectric effect provides evidence that energy is quantized. In order to establish the particle nature of radiation, it is necessary that photons must carry momentum. In 1922, Arthur Compton studied the scattering of x-rays of known frequency from graphite and looked at the recoil electrons and the scattered x-rays. According to wave theory, when an electromagnetic wave of frequency is incident on an atom, it would cause electrons to oscillate. The electrons would absorb energy from the wave and re-radiate electromagnetic wave of a frequency . The frequency of scattered radiation would depend on the amount of energy absorbed from the wave, i.e. on the intensity of incident radiation and the duration of the exposure of electrons to the radiation and not on the frequency of the incident radiation. Compton found that the wavelength of the scattered radiation does not depend on the intensity of incident radiation but it depends on the angle of scattering and the wavelength of the incident beam. The wavelength of the radiation scattered at an angle is given by .where is the rest mass of the electron. The constant is known as the Compton wavelength of the electron and it has a value 0.0024 nm. The spectrum of radiation at an angle consists of two peaks, one at and the other at . -

1 the Principle of Wave–Particle Duality: an Overview
3 1 The Principle of Wave–Particle Duality: An Overview 1.1 Introduction In the year 1900, physics entered a period of deep crisis as a number of peculiar phenomena, for which no classical explanation was possible, began to appear one after the other, starting with the famous problem of blackbody radiation. By 1923, when the “dust had settled,” it became apparent that these peculiarities had a common explanation. They revealed a novel fundamental principle of nature that wascompletelyatoddswiththeframeworkofclassicalphysics:thecelebrated principle of wave–particle duality, which can be phrased as follows. The principle of wave–particle duality: All physical entities have a dual character; they are waves and particles at the same time. Everything we used to regard as being exclusively a wave has, at the same time, a corpuscular character, while everything we thought of as strictly a particle behaves also as a wave. The relations between these two classically irreconcilable points of view—particle versus wave—are , h, E = hf p = (1.1) or, equivalently, E h f = ,= . (1.2) h p In expressions (1.1) we start off with what we traditionally considered to be solely a wave—an electromagnetic (EM) wave, for example—and we associate its wave characteristics f and (frequency and wavelength) with the corpuscular charac- teristics E and p (energy and momentum) of the corresponding particle. Conversely, in expressions (1.2), we begin with what we once regarded as purely a particle—say, an electron—and we associate its corpuscular characteristics E and p with the wave characteristics f and of the corresponding wave. -

Chapter 09584
Author's personal copy Excitons in Magnetic Fields Kankan Cong, G Timothy Noe II, and Junichiro Kono, Rice University, Houston, TX, United States r 2018 Elsevier Ltd. All rights reserved. Introduction When a photon of energy greater than the band gap is absorbed by a semiconductor, a negatively charged electron is excited from the valence band into the conduction band, leaving behind a positively charged hole. The electron can be attracted to the hole via the Coulomb interaction, lowering the energy of the electron-hole (e-h) pair by a characteristic binding energy, Eb. The bound e-h pair is referred to as an exciton, and it is analogous to the hydrogen atom, but with a larger Bohr radius and a smaller binding energy, ranging from 1 to 100 meV, due to the small reduced mass of the exciton and screening of the Coulomb interaction by the dielectric environment. Like the hydrogen atom, there exists a series of excitonic bound states, which modify the near-band-edge optical response of semiconductors, especially when the binding energy is greater than the thermal energy and any relevant scattering rates. When the e-h pair has an energy greater than the binding energy, the electron and hole are no longer bound to one another (ionized), although they are still correlated. The nature of the optical transitions for both excitons and unbound e-h pairs depends on the dimensionality of the e-h system. Furthermore, an exciton is a composite boson having integer spin that obeys Bose-Einstein statistics rather than fermions that obey Fermi-Dirac statistics as in the case of either the electrons or holes by themselves. -

1. Physical Constants 1 1
1. Physical constants 1 1. PHYSICAL CONSTANTS Table 1.1. Reviewed 1998 by B.N. Taylor (NIST). Based mainly on the “1986 Adjustment of the Fundamental Physical Constants” by E.R. Cohen and B.N. Taylor, Rev. Mod. Phys. 59, 1121 (1987). The last group of constants (beginning with the Fermi coupling constant) comes from the Particle Data Group. The figures in parentheses after the values give the 1-standard- deviation uncertainties in the last digits; the corresponding uncertainties in parts per million (ppm) are given in the last column. This set of constants (aside from the last group) is recommended for international use by CODATA (the Committee on Data for Science and Technology). Since the 1986 adjustment, new experiments have yielded improved values for a number of constants, including the Rydberg constant R∞, the Planck constant h, the fine- structure constant α, and the molar gas constant R,and hence also for constants directly derived from these, such as the Boltzmann constant k and Stefan-Boltzmann constant σ. The new results and their impact on the 1986 recommended values are discussed extensively in “Recommended Values of the Fundamental Physical Constants: A Status Report,” B.N. Taylor and E.R. Cohen, J. Res. Natl. Inst. Stand. Technol. 95, 497 (1990); see also E.R. Cohen and B.N. Taylor, “The Fundamental Physical Constants,” Phys. Today, August 1997 Part 2, BG7. In general, the new results give uncertainties for the affected constants that are 5 to 7 times smaller than the 1986 uncertainties, but the changes in the values themselves are smaller than twice the 1986 uncertainties. -

The Graviton Compton Mass As Dark Energy
Gravitation, Mathematical Physics and Field Theory Revista Mexicana de F´ısica 67 040703 1–5 JULY-AUGUST 2021 The graviton Compton mass as dark energy T. Matosa and L. L.-Parrillab aDepartamento de F´ısica, Centro de Investigacion´ y de Estudios Avanzados del IPN, Apartado Postal 14-740, 07000, CDMX, Mexico.´ bInstituto de Ciencias Nucleares, Universidad Nacional Autonoma´ de Mexico,´ Circuito Exterior C.U., Apartado Postal 70-543, 04510, CDMX, Mexico.´ Received 3 January 2021; accepted 18 February 2021 One of the greatest challenges of science is to understand the current accelerated expansion of the Universe. In this work, we show that by considering the quantum nature of the gravitational field, its wavelength can be associated with an effective Compton mass. We propose that this mass can be interpreted as dark energy, with a Compton wavelength given by the size of the observable Universe, implying that the dark energy varies depending on this size. If we do so, we find that: 1.- Even without any free constant for dark energy, the evolution of the Hubble parameter is exactly the same as for the LCDM model, so this model has the same predictions as LCDM. 2.- The density rate of the dark energy is ¤ = 0:69 which is a very similar value as the one found by the Planck satellite ¤ = 0:684. 3.- The dark energy has this value because it corresponds to the actual size of the radius of the Universe, thus the coincidence problem has a very natural explanation. 4.- It, is possible to find also a natural explanation to why observations inferred from the local distance ladder find the value H0 = 73 km/s/Mpc for the Hubble constant.