Genomics Analysis of Aphanomyces Spp. Identifies a New Class of Oomycete Effector Associated with Host Adaptation Elodie Gaulin1* , Michiel J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Identification of Fungi
Molecular Identification of Fungi Youssuf Gherbawy l Kerstin Voigt Editors Molecular Identification of Fungi Editors Prof. Dr. Youssuf Gherbawy Dr. Kerstin Voigt South Valley University University of Jena Faculty of Science School of Biology and Pharmacy Department of Botany Institute of Microbiology 83523 Qena, Egypt Neugasse 25 [email protected] 07743 Jena, Germany [email protected] ISBN 978-3-642-05041-1 e-ISBN 978-3-642-05042-8 DOI 10.1007/978-3-642-05042-8 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2009938949 # Springer-Verlag Berlin Heidelberg 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: WMXDesign GmbH, Heidelberg, Germany, kindly supported by ‘leopardy.com’ Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Dedicated to Prof. Lajos Ferenczy (1930–2004) microbiologist, mycologist and member of the Hungarian Academy of Sciences, one of the most outstanding Hungarian biologists of the twentieth century Preface Fungi comprise a vast variety of microorganisms and are numerically among the most abundant eukaryotes on Earth’s biosphere. -
Published Version
PUBLISHED VERSION Rays H. Y. Jiang ... Vincent Bulone ... et al. Distinctive expansion of potential virulence genes in the genome of the oomycete fish pathogen Saprolegnia parasitica PLoS Genetics, 2013; 9(6):e1003272-1-e1003272-20 © 2013 Jiang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Originally published at: http://doi.org/10.1371/journal.pgen.1003272 PERMISSIONS http://creativecommons.org/licenses/by/4.0/ http://hdl.handle.net/2440/97179 Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen Saprolegnia parasitica Rays H. Y. Jiang1., Irene de Bruijn2¤a., Brian J. Haas1., Rodrigo Belmonte2,3,LarsLo¨ bach2, James Christie2,3, Guido van den Ackerveken4, Arnaud Bottin5, Vincent Bulone6, Sara M. Dı´az-Moreno6, Bernard Dumas5, Lin Fan1, Elodie Gaulin5, Francine Govers7,8, Laura J. Grenville-Briggs2,6, Neil R. Horner2, Joshua Z. Levin1, Marco Mammella9, Harold J. G. Meijer7, Paul Morris10, Chad Nusbaum1, Stan Oome4, Andrew J. Phillips2, David van Rooyen2, Elzbieta Rzeszutek6, Marcia Saraiva2, Chris J. Secombes3, Michael F. Seidl8,11, Berend Snel8,11, Joost H. M. Stassen4, Sean Sykes1, Sucheta Tripathy12, Herbert van den Berg2, Julio C. Vega-Arreguin13, Stephan Wawra2, Sarah K. Young1, Qiandong Zeng1, Javier Dieguez- Uribeondo14, Carsten Russ1", Brett M. Tyler12¤b", Pieter van West2*" 1 Broad Institute -

Host-Pathogen Interactions in Root Infecting Oomycete Species
Host-Pathogen Interactions in Root Infecting Oomycete Species Sara Hadji Mollahossein Faculty of Natural Resources and Agricultural Sciences Department of Forest Mycology and Plant Pathology Uppsala Doctoral Thesis Swedish University of Agricultural Sciences Uppsala 2015 Acta Universitatis Agriculturae Sueciae 2015:9 Cover: Pea plant infected with Phytophthora pisi (left), control plant (right). Sporangia releasing zoospores (left) and oospores (right) in infected root tissue. (Photo: Sara Hosseini) ISSN 1652-6880 ISBN (print version) 978-91-576-8216-1 ISBN (electronic version) 978-91-576-8217-8 © 2015 Sara Hadji Mollahossein, Uppsala Print: SLU Service/Repro, Uppsala 2015 Host-Pathogen Interactions in Root Infecting Oomycete Species Abstract The oomycetes include some of the most devastating pathogens on both cultivated crops and wild plants. In the genus Phytophthora some closely related species have a broad host range, while others are very host specific. The aim of this project was to gain an understanding of the mechanisms underlying the differentiation of a subgroup of root-infecting Phytophthora species and to gain knowledge about the plant immune responses triggered by distantly related oomycetes that adapted to the same legume host. We investigated the zoospore chemotaxis of legume-root infecting Phytophthora species to different isoflavonoid compounds and explored a possible connection to host preference. Our results showed that specific chemotaxis towards host isoflavones is of limited importance in Phytophthora sojae and Phytophthora vignae, while, specific chemotaxis of Phytophthora pisi and Phytophthora niederhauserii indicated an adaptation to their pathogenicity on the host and lack of pathogenicity on non-host plants. The comparative proteomic study of P. pisi and P. -

The Taxonomy and Biology of Phytophthora and Pythium
Journal of Bacteriology & Mycology: Open Access Review Article Open Access The taxonomy and biology of Phytophthora and Pythium Abstract Volume 6 Issue 1 - 2018 The genera Phytophthora and Pythium include many economically important species Hon H Ho which have been placed in Kingdom Chromista or Kingdom Straminipila, distinct from Department of Biology, State University of New York, USA Kingdom Fungi. Their taxonomic problems, basic biology and economic importance have been reviewed. Morphologically, both genera are very similar in having coenocytic, hyaline Correspondence: Hon H Ho, Professor of Biology, State and freely branching mycelia, oogonia with usually single oospores but the definitive University of New York, New Paltz, NY 12561, USA, differentiation between them lies in the mode of zoospore differentiation and discharge. Email [email protected] In Phytophthora, the zoospores are differentiated within the sporangium proper and when mature, released in an evanescent vesicle at the sporangial apex, whereas in Pythium, the Received: January 23, 2018 | Published: February 12, 2018 protoplast of a sporangium is transferred usually through an exit tube to a thin vesicle outside the sporangium where zoospores are differentiated and released upon the rupture of the vesicle. Many species of Phytophthora are destructive pathogens of especially dicotyledonous woody trees, shrubs and herbaceous plants whereas Pythium species attacked primarily monocotyledonous herbaceous plants, whereas some cause diseases in fishes, red algae and mammals including humans. However, several mycoparasitic and entomopathogenic species of Pythium have been utilized respectively, to successfully control other plant pathogenic fungi and harmful insects including mosquitoes while the others utilized to produce valuable chemicals for pharmacy and food industry. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

Connecticut Aquatic Nuisance Species Management Plan
CONNECTICUT AQUATIC NUISANCE SPECIES MANAGEMENT PLAN Connecticut Aquatic Nuisance Species Working Group TABLE OF CONTENTS Table of Contents 3 Acknowledgements 5 Executive Summary 6 1. INTRODUCTION 10 1.1. Scope of the ANS Problem in Connecticut 10 1.2. Relationship with other ANS Plans 10 1.3. The Development of the CT ANS Plan (Process and Participants) 11 1.3.1. The CT ANS Sub-Committees 11 1.3.2. Scientific Review Process 12 1.3.3. Public Review Process 12 1.3.4. Agency Review Process 12 2. PROBLEM DEFINITION AND RANKING 13 2.1. History and Biogeography of ANS in CT 13 2.2. Current and Potential Impacts of ANS in CT 15 2.2.1. Economic Impacts 16 2.2.2. Biodiversity and Ecosystem Impacts 19 2.3. Priority Aquatic Nuisance Species 19 2.3.1. Established ANS Priority Species or Species Groups 21 2.3.2. Potentially Threatening ANS Priority Species or Species Groups 23 2.4. Priority Vectors 23 2.5. Priorities for Action 23 3. EXISTING AUTHORITIES AND PROGRAMS 30 3.1. International Authorities and Programs 30 3.2. Federal Authorities and Programs 31 3.3. Regional Authorities and Programs 37 3.4. State Authorities and Programs 39 3.5. Local Authorities and Programs 45 4. GOALS 47 3 5. OBJECTIVES, STRATEGIES, AND ACTIONS 48 6. IMPLEMENTATION TABLE 72 7. PROGRAM MONITORING AND EVALUATION 80 Glossary* 81 Appendix A. Listings of Known Non-Native ANS and Potential ANS in Connecticut 83 Appendix B. Descriptions of Species Identified as ANS or Potential ANS 93 Appendix C. -

Synopsis of Freshwater Crayfish Diseases and Commensal Organisms Brett .F Edgerton James Cook University, [email protected]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Parasitology, Harold W. Manter Laboratory of Laboratory of Parasitology 3-2002 Synopsis of Freshwater Crayfish Diseases and Commensal Organisms Brett .F Edgerton James Cook University, [email protected] Louis H. Evans Curtin University of Technology Frances J. Stephens Curtin University of Technology Robin M. Overstreet Gulf Coast Research Laboratory, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Aquaculture and Fisheries Commons, and the Parasitology Commons Edgerton, Brett .;F Evans, Louis H.; Stephens, Frances J.; and Overstreet, Robin M., "Synopsis of Freshwater Crayfish Diseases and Commensal Organisms" (2002). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 884. https://digitalcommons.unl.edu/parasitologyfacpubs/884 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Aquaculture 206:1–2 (March 2002), pp. 57–135; doi: 10.1016/S0044-8486(01)00865-1 Copyright © 2002 Elsevier Science. Creative Commons Attribution Non-Commercial No Deriva- tives License. Accepted October 18, 2001; published online November 30, 2001. Synopsis of Freshwater Crayfish Diseases and Commensal Organisms Brett F. Edgerton,1 Louis H. Evans,2 Frances J. Stephens,2 and Robin M. Overstreet3 1. Department of Microbiology and Immunology, James Cook University, Townsville, QLD 4810, Australia 2. -

Genetic Mapping of Resistance to Aphanomyces Root Rot in Alfalfa *E
Genetic mapping of resistance to Aphanomyces root rot in alfalfa15 Samac, D.A.*, Dornbusch, M. R., Bucciarelli, B., Miller, S. S. and Yu, L.-X. USDA-Agricultural Research Service *e-mail: [email protected] KEYWORDS: Aphanomyces euteiches, genotyping by sequencing, NBS-LRR resistance genes Aphanomyces root rot (ARR), caused by the oomycete Aphanomyces euteiches, is one of the most important yield-limiting factors in production of legumes. In Europe, it is the main limiting factor for pea production while in the United States it is one of the most important diseases of alfalfa and pea (Gaulin et al., 2007). The disease causes stunting and death of alfalfa seedlings with complete stand loss occurring in wet and poorly drained soils. Non-lethal damage to seedling roots reduces forage yields and winter survival. The pathogen can infect adult plants during wet periods to cause loss of feeder roots and root nodules, reducing nitrogen fixation, forage yield, and stand life. Varieties with resistance to ARR became widely available in the 1990s; however, failure of resistant varieties identified a second race of the pathogen. Recent reports of failure of varieties with resistance to both race 1 and race 2 suggest that additional pathogenic races are present in alfalfa production fields. The goals of this research were to: (i) determine the prevalence of race 1 and race 2 strains in Minnesota and New York; (ii) identify novel strains of the pathogen; (iii) characterize the race-specific resistance to Aphanomyces root rot; and (iv) map resistance genes in order to facilitate breeding for resistance and to clarify race/resistance gene relationships. -

Development of New Genome-Informed Genotyping Tools for Aphanomyces Astaci
Development of new genome-informed genotyping tools for Aphanomyces astaci Submitted by Diana Minardi to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Biological Sciences In May 2017 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. Signature: ………………………………………………………….. 1 Acknowledgements I would like to sincerely thank my supervisors Dr Mark van der Giezen, Dr David Studholme, and Dr Birgit Oidtmann for their invaluable guidance and support during these 3 years (plus a bit) of PhD, both practically and in the writing of the thesis. I would like to thank Cefas and the University of Exeter for funding the project and for giving me the opportunity to embrace this challenge and adventure. I would like to thank all of the University of Exeter “Biocat” lab and office, with whom I’ve spent most of my coffee time. But especially Mirella (for the laughs and cries) and Simone (for the sweaty runs around campus). I would also like to thank Chiara for her constant support, encouragement, and beer. Finally, I would like to thank Tony, for his patience, and my family, for their “overseas” support: I’ve finally finished “the shrimp” book! 2 Abstract Aphanomyces spp. are water moulds, eukaryotic fungus-like organisms, belonging to the class Oomycota. -

Crayfish Plague Epizootics in Germany-Classification of Two
DISEASES OF AQUATIC ORGANISMS Vol. 35: 235-238,1999 Published February 26 Dis Aquat Org NOTE Crayfish plague epizootics in Germany-classification of two German isolates of the crayfish plague fungus Aphanomyces astaci by random amplification of polymorphic DNA Birgit Oidtmann1,*,Lage Cerenius2,Ines Schmidl, Rudolf ~offmann',Kenneth soderhal12 '~nstituteof Zoology, Fish Biology and Fish Diseases. University of Munich, Kaulbachstr. 37, D-80539 Munich, Germany '~ivisionof Physiological Mycology, University of Uppsala, Villavigen 6. S-752 36 Uppsala, Sweden ABSTRACT: Following 2 outbreaks of crayfish plague m cumb to infection. During the past century, the distrib- southern Germany, the causative agent, the oomycete fungus ution of non-native crayfish species has dramatically Aphanomyces astaci, was isolated from the diseased Astacus increased due to stocking activities, the natural spread astacus. The identity of the 2 strains was confirmed using established techniques, such as physiology, spore production of stocked populations, and the release of exotic and the fact that the isolated strains were hlghly virulent for crayfish by private aquarist or pond owners. In areas, A. astacus in infection experiments. The relationship between where American species have been introduced, mass these German strains and other A astaci strains was In- mortalities of European crayfish have frequently been vestigated using randomly amplified polymorphic DNA-poly- merase chain reaction (RAPD-PCR).The German strains were documented (Alderman 1996). found to be closely related to a strain that had been isolated Mass mortalities of Astacus astacus were observed in from PacLfastacus len~usculusfrom Lake Tahoe, USA. 1996 in 3 independent sites in southern Germany sep- arated by at least 80 km. -

Root Rots in Pulses
CROP DEVELOPMENT CENTRE Root Rots in Pulses Michelle Hubbard, Agriculture and Agri-Food Canada, Swift Current Research Scientist, Pulse Pathology Co-authors: Sabine Banniza, Zakir Hossain, Sherrilyn Phelps, Syama Chatterton, Luke Bainard & Barb Ziesman What is root rot? Caused by micro-organisms Range of pathogens • Fusarium species • Pythium species • Rhizoctonia solani • Aphanomyces euteiches Pea and lentil do not like wet feet High soil moisture can cause • ↓ root and shoot growth • yellowing • ↓ nodulation Stressed plants are more susceptible to infection Normal soil Water- moisture saturated Peas in sterile field soil Root rot is a complex Fusarium species Pythium species Rhizoctonia solani Aphanomyces euteiches And it is complicated! Wider host range True fungi • Fusarium spp. (e.g. solani, • Fusarium spp. avenaceum, acuminatum, graminearum) • Rhizoctonia solani • Rhizoctonia solani • Pythium spp. Fungus-like organisms (oomycetes) Attack only specific plants: • Pythium spp. • Fusarium oxysporum f.sp. pisi, f.sp. ciceris or f. sp. • Aphanomyces lentis, F. virgulifome euteiches • Aphanomyces euteiches • Phytophthora spp. • Phytophthora spp Fusarium Courtesy of S. Chatterton, AAFC Infects many different plants Courtesy of F. Dokken-Bouchard, SMA Aphanomyces Oospores Infects pea and lentil Oospores = resting spores • More vulnerable after they germinate Zoospores: can swim short distances Courtesy of S. Chatterton, AAFC Courtesy of F. Dokken-Bouchard, SMA Aphanomyces Oospores EveryInfects time pea aand plant lentil gets infected,Oospores -
Meeting Program
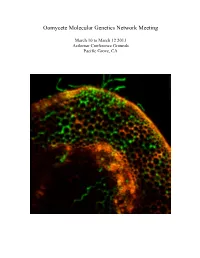
Oomycete Molecular Genetics Network Meeting March 10 to March 12 2013 Asilomar Conference Grounds Pacific Grove, CA Oomycete Molecular Genetics Network Meeting March 10 to March 12, 2013 Asilomar Conference Grounds Pacific Grove, CA The Oomycete Molecular Genetics Research Network was initially funded by an NSF Research Coordination Network grant in 2001. The purpose of our annual meeting is to promote communication and collaboration, and minimize duplication of effort within the worldwide Oomycete molecular genetics community. The oomycete molecular genetics community now numbers well over one hundred labs from across the world, and papers on oomycetes attract a readership that goes well beyond the community itself. The Oomycete Molecular Genetics Conference alternates between USA and Eurasia, and returns this year to Asilomar, California. This year’s meeting will cover some of the latest research on Effector Biology, Genomics and Oomycete evolution and population biology. With over 100 attendees expected, this meeting represents the largest network meeting held in the US. Committee Chairs John McDowell Mark Gijzen Associate Professor Research Scientist Plant Pathology, Physiology and Weed Science Agriculture and Agri-food Canada Virginia Tech, Blacksburg, VA 24061 London ON, Canada NSV4T3 Meeting Logistics Facilities Coordinator Joel Shuman Paul Morris Project Manager Professor, Biological Sciences Plant Pathology, Physiology and Weed Science Bowling Green State University Virginia Tech, Blacksburg, VA24061 Bowling Green, OH 43403 Cover: Confocal image of GFP-expressing Phytophthora sojae at 12h after inoculation of soybean hypocotyls. Image courtesy of Kai-Tao from the lab of Yuanchao Wang, Nanjing Agricultural University, Nanjing, China. Meeting Sponsors This meeting is supported in part by NSF grant MCB-0639226 to Brett Tyler OMGN 2013 Program Location: Talks will be held at Kiln on Sunday Morning and at Fred Farr for all other sessions.