SECURE®-C Cervical Artificial Disc Is A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Broaching Solutions for Every Application Hassay Savage Has the Most Comprehensive Program of Standard Broaching Solutions for All Applications
IN THIS BOOK... Hassay Savage PRECISION BROACHES STANDARD & CUSTOM BROACHING SOLUTIONS FOR EVERY APPLICATION Hassay Savage has the most comprehensive program of standard broaching solutions for all applications. We offer Custom Broaching Solutions for all your broaching needs. Let us be your partner in your unique, cutting applications! GMauvaisUSA QUALITY MICRO DRILLS superior precision • quality • consistency The most consistent quality micro drill program in the world, GMauvais has a complete line of HSS-E COBALT and Solid Micro Grain Carbide MICRO Drills. Standard sizes range from .1mm to 3mm, with 1,000 line items in stock. Custom made micro drills for your special application and fast delivery is our specialty. ® UNIQUE ROUND CUTTING TOOLS performance • innovation • specialization The world’s finest Center Drill, NC Spot Drill, Countersink, Multi-V, Micro Reaming and End Mill Cutting Tools for your most challenging cutting tool applications. We hope you enjoy using our outstanding collection of the finest cutting tools for virtually every cutting application. Hassay Savage Company • 3 Great Programs, One Great Company! Hassay Savage Let us be your partner in unique cutting applications! www.hassay-savage.com 6130 6220 6230 1100 3200 TM GMauvaisUSA TM GMA HaASSuAY SAvVAGaE COiMPsANYUSA A HASSAY SAVAGE COMPANY 5140 3100 5100 6140 6120 NEW Items for 2017 6100 6200º www.hassay-savage.com Unique, Precision NEW Items for 2017 Cutting Tools! NEW 8760 Series Blind Hole Reamers w/Coolant www.hassay-savage.com What is Broaching? Broaching is a progressive metal-cutting process which incorporates a series of roughing, semi-finishing, and finishing teeth designed to remove successive portions of stock as the tool moves through or across a workpiece in a one-pass linear operation. -
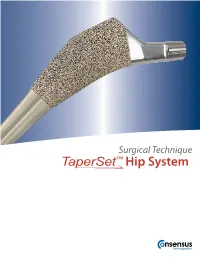
Surgical Technique
Surgical Technique Hip System INDICATIONS FOR USE The TaperSet™ Hip System is designed for total or partial hip arthroplasty and is intended to be used with compat- ible components of the Consensus Hip System. The indications for use are: A. Significantly impaired joints resulting from rheumatoid, osteo, and post-traumatic arthritis. B. Revision of failed femoral head replacement, cup arthroplasty or other hip procedures. C. Proximal femoral fractures. D. Avascular necrosis of the femoral head. E. Non-union of proximal femoral neck fractures. F. Other indications such as congenital dysplasia, arthrodesis conversion, coxa magna, coxa plana, coxa vara, coxa valga, developmental conditions, metabolic and tumorous conditions, osteomalacia, osteoporosis, pseudarthrosis conversion, and structural abnormalities. The TaperSet™ hip stem is indicated for cementless use. Hip System Surgical Technique Table of Contents Introduction ......................................................................................................................................................................1 Templating / Preoperative Planning .........................................................................................................................2 Patient Positioning / Surgical Approach .................................................................................................................2 Acetabular Preparation and Implantation .............................................................................................................2 -

Inclusion Characteristics and Their Link to Tool Wear in Metal Cutting of Clean Steels Suitable for Automotive Applications
Inclusion Characteristics and Their Link to Tool Wear in Metal Cutting of Clean Steels Suitable for Automotive Applications Niclas Ånmark Licentiate Thesis Stockholm 2015 Division of Applied Process Metallurgy Department of Materials Science and Engineering School of Industrial Engineering and Management KTH Royal Institute of Technology SE-100 44 Stockholm, Sweden Akademisk avhandling som med tillstånd av Kungliga Tekniska Högskolan i Stockholm, framlägges för offentlig granskning för avläggande av Teknologie Licentiatexamen, tisdagen den 5 maj 2015, kl. 10.00 i Sefströmsalen, M131, Brinellvägen 23, Kungliga Tekniska Högskolan, Stockholm. ISBN 978-91-7595-505-6 Niclas Ånmark: Inclusion Characteristics and Their Link to Tool Wear in Metal Cutting of Clean Steels Suitable for Automotive Applications Division of Applied Process Metallurgy Department of Materials Science and Engineering School of Industrial Engineering and Management KTH Royal Institute of Technology SE-100 44 Stockholm, Sweden ISBN 978-91-7595-505-6 Cover: Reprinted with permission of Sandvik Coromant © Niclas Ånmark Abstract This thesis covers some aspects of hard part turning of carburised steels using a poly-crystalline cubic boron nitride (PCBN) cutting tool during fine machining. The emphasis is on the influence of the steel cleanliness and the characteristics of non-metallic inclusions in the workpiece on the active wear mechanisms of the cutting tool. Four carburising steel grades suitable for automotive applications were included, including one that was Ca-treated. A superior tool life was obtained when turning the Ca-treated steel. The superior machinability is associated with the deposition of lubricating (Mn,Ca)S and (CaO)x-Al2O3-S slag layers, which are formed on the rake face of the cutting tool during machining. -

Band Saw Blades2021 Welcome Letter from Simonds President
THE PROFESSIONALS’ EDGE™ www.simondssaw.com Band Saw Blades2021 Welcome letter from Simonds President Thank you for choosing Simonds. It is our Mission to empower the skilled masters of the metal fabricating industry with cutting edge technologies and the science and knowledge of metal cutting so that you can make great products for your customers. We’ve been providing industry all over the world with a better way to cut for over 180 years. We are the teachers of the metal cutting industry and we are ready to help you be the best you can be. Our blade products are produced to the highest standards by our 2 world class factories in Melsungen Germany and Louisville Kentucky USA. Thanks again for choosing us. We are excited to have you as a partner. David Miles President Table of Contents History of Simonds 1832-2020 4 Band Applications Cross Reference Chart 10 Tooth Pitch Selector 11 Break-in & Blade Terminology 12 Sawing Variables 13 Speed and Feed Chart 14 Sinewave 16 Flat Tube Tube Solid Solid Tubes H Beams I Beams Bundles Pallets Bundle CARBIDE BANDSAW BLADES Triple Chip 18 QG7 19 TCi22 20 CHM 21 Set Tooth 22 BI-METAL BANDSAW BLADES Epic GP 24 SBX GP 26 SBX ONE 27 Siclone 28 RS Pro 29 Pallet Buster 30 CARBON BANDSAW BLADES Flex Back 32 Wood Max 32 Hard Back 33 Area Calculator 34 Other SIMONDS products 35 THE PROFESSIONALS’ EDGE™ www.simondssaw.com 1878 As the agricultural market base moves further west, the mower blade and reaper business is sold off in 1878. -

Water Jet Machining (WJM)
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 06 Issue: 12 | Dec 2019 www.irjet.net p-ISSN: 2395-0072 Advance Manufacturing Processes Review Part II: Water Jet Machining (WJM) Swapnil Umredkar1, Vallabh Bhoyar2 1,2UG Student, Mechanical Engineering Department, G. H. Raisoni College of Engineering, Maharashtra, India -------------------------------------------------------------------------***------------------------------------------------------------------------ Abstract – Water jet has been in existence and practised cutting tool material. For such conditions, inspite of for over twenty years, but it is yet to reach its full potential recent developments, traditional methods of machining in the construction and manufacturing industry. The are uneconomical, time consuming and degree of paper presents aspects regarding an innovative accuracy and surface finish and poor. The newer nonconventional technology. Many research works have machine processes, so developed are often called been done in the field of Water Jet Machining; however, ‘Modern Machining Methods’ or non–traditional these is always a scope for research in any particular field. machining processes or unconventional machining Many research works have been put forward on the basis processes. These are unconventional in the sense that of changing the process parameter also many have been conventional tool is not utilize for cutting the material. It put forward by the use of optimization techniques, etc employs some form energy like mechanical, chemical, thermal, electro-chemical etc. for cutting the material. Keywords — water jet, abrasive, nonconventional, Also, the absence of tool workpiece contact or relative technological, construction, innovative, industry motion, makes the processes a non-traditional one. 1. INTRODUCTION 1.1Definition of non-traditional machining process Machining process removes certain parts of the work It is defined as a group of processes that removes excess piece to change them to final product. -

Woodworking Tools HISTORY and PRESENT TIME
Woodworking tools HISTORY AND PRESENT TIME HISTORY The tool production in Hulin began in 1934. The firm was founded by Mr. Stu- deník who named the new company “First Moravian factory for saws and tools“. At first the company started producing hand saws, circular saw blades and was gradually enriching the production programme with cutters for wood cutting and other tools for wood working. In the 1960th the production assortment enriched with TCT circular saw blades, gang saw blades, planer knives, machine knives, metal cutting tools and saw bodies. PRESENT TIME PILANA TOOLS with about 600 workers is in the process of dynamic develop- ment and is one of the biggest producers of tools in Europe. The tools are made of the best-quality steel in accordance with DIN and ISO standards. The qual- ity is closely watched at each production stage. For the highest precision the most up-to-date equipment is used: Laser, CNC grinding machines, CNC milling machines, CNC sharpening machines, automatic furnaces and other automatic and semiautomatic machinery. The constant attention is paid to the production improvement and automation. These measures, together with long-lasting experience and low costs, enable to offer high quality products at competitive prices. PILANA TOOLS regularly exports 80% of its products to over 70 countries world-wide. PILANA TOOLS consists of property-joined companies: PILANA TOOLS a.s. PILANA TOOLS Wood Saws spol. s.r.o. PILANA TOOLS Saw Bodies spol. s.r.o. PILANA TOOLS Metal spol. s.r.o. PILANA TOOLS Knives spol. s.r.o. INDEX -

Guide to Machining Carpenter Specialty Alloys Guide to Machining Carpenter Special Ty All O Ys
GUIDE TO MACHINING CARPENTER SPECIAL GUIDE TO MACHINING CARPENTER SPECIALTY ALLOYS Carpenter Technology Corporation TY ALL Wyomissing, PA 19610 U.S.A. 1-800-654-6543 Visit us at www.cartech.com O YS For on-line purchasing in the U.S., visit www.carpenterdirect.com GUIDE TO MACHINING CARPENTER SPECIALTY ALLOYS Carpenter Technology Corporation Wyomissing, Pennsylvania 19610 U.S.A. Copyright 2002 CRS Holdings, Inc. All Rights Reserved. Printed in U.S.A. 9-02/7.5M The information and data presented herein are suggested starting point values and are not a guarantee of maximum or minimum values. Applications specifically suggested for material described herein are made solely for the purpose of illustration to enable the reader to make his/her own evaluation and are not intended as warranties, either express or implied, of fitness for these or other purposes. There is no representation that the recipient of this literature will receive updated editions as they become available. Unless otherwise noted, all registered trademarks are property of CRS Holdings, Inc., a subsidiary of Carpenter Technology Corporation. ISO 9000 and QS-9000 Registered Headquarters - Reading, PA Guide to Machining CARPENTER SPECIALTY ALLOYS Contents Introduction ............................................................................. 1 General Stainless Material and Machining Characteristics .... 3 Classification of Stainless Steels .............................................. 5 Basic Families and Designations ........................................ 5 Austenitic -

Opportunities of Efficiency Improvement by the Use of Hydro Lubricants
GEAR SOLUTIONS MAGAZINE Your Resource for Machines, Services, and Tooling for the Gear Industry OPPORTUNITIES OF EFFICIENCY IMPROVEMENT BY THE USE HYDRO LUBRICANTS ISSUE FOCUS Lubrication | Broaching OPPORTUNITIES OF EFFICIENCY IMPROVEMENT BY THE USE OF HYDRO LUBRICANTS COMPANY PROFILE NEUGART MARCH 2020 MARCH 2020 gearsolutions.com TRUSTEDIN THE MOST HOSTILE ENVIRONMENTS. LOOKING FOR AN OUT OF THIS WORLD CAREER? If you’d like to be part of a world-class gear manufacturing Excellence company that values and invests in its people, and you without have what it takes to deliver Excellence Without Exception… exception. Forest City Gear is looking to add team members in: • Machining/Manufacturing • Engineering When there is absolutely no room for error, • Supervision • Sales we deliver extreme accuracy, reliability, and • Quality Assurance unyielding consistency. forestcitygear.com 815.623.2168 FCG_GearSolutionsFullPageAd_WKG.indd 1 1/15/20 3:19 PM www.toolink-eng.com 6595 Odell Place, Suite I Boulder, CO 80301 303-776-6212 ARE YOU MAXIMIZING YOUR EXPOSURE? Connect your company to the gear industry with a storefront in the Gear Solutions Community. Storefronts paint a portrait of your company with a 500-word description and include your logo, website link, phone number, email addresses, and videos. Your social media pages such as Twitter and Facebook are integrated with live updates, which may also be re-posted through our social media feeds. With a community storefront, your company also receives a premium listing in the annual Buyer’s Guide published each November. Premium listings feature graphic treatments to draw more attention to your company. JOIN THE GEAR SOLUTIONS For information on how you can participate in the COMMUNITY GearSolutions.com community storefront, contact FOR ONLY [email protected]. -

Precision Tools
Precision Tools Precision Tools Gear Cutting Tools & Broaches Pursuing advanced high-speed technology that is both user and environmentally friendly Since developing Japan's first broaching machine in the late 1920s, Fujikoshi has developed a variety of tools and machine tools to handle advancements in production systems. Fujikoshi continues to lead the way by developing machining systems that integrate tools and machines. Pursuing advanced high-speed technology that is both user and environmentally friendly Since developing Japan's first broaching machine in the late 1920s, Fujikoshi has developed a variety of tools and machine tools to handle advancements in production systems. Fujikoshi continues to lead the way by developing machining systems that integrate tools and machines. ndex Gear Cutting Tools Gear Cutting Comparison and Types 25 Broaches Design of Broach ools Guidance NACHI Accuracy of Gear Shaper Cutters 26 Technical Introduction Basic Design and Cutting Method 61 Materials and Coating of Gear Cutting Tools 5 Cutting Condition and Regrinding 27 Hard Broaches 45 Calculation of Pulling Load 62 Gear Cutting T Disk Type Shaper Cutters Type1Standard Dimensions 28 Broach for MQL 46 Face Angle and Relief Angle 63 Technical Introduction Disk Type Shaper Cutters Type2Standard Dimensions 30 Off-normal Gullet Helical Broach 47 Finished Size of Broaches 64 Hard Hobbing 6 Disk Type Shaper Cutters Type2Standard Dimensions 31 Micro Module Broaching 48 Helical Gear Shaper Cutters High Speed Dry Hobbing 7 Essential Points and Notice 65 Disk -

Cutting Fluid Management: Small Machining Operations
University of Northern Iowa UNI ScholarWorks Iowa Waste Reduction Center Book Gallery Iowa Waste Reduction Center 2003 Cutting Fluid Management: Small Machining Operations Iowa Waste Reduction Center Let us know how access to this document benefits ouy Copyright ©2003 Iowa Waste Reduction Center Follow this and additional works at: https://scholarworks.uni.edu/iwrc_facbook Part of the Environmental Sciences Commons Recommended Citation Iowa Waste Reduction Center, "Cutting Fluid Management: Small Machining Operations" (2003). Iowa Waste Reduction Center Book Gallery. 12. https://scholarworks.uni.edu/iwrc_facbook/12 This Book is brought to you for free and open access by the Iowa Waste Reduction Center at UNI ScholarWorks. It has been accepted for inclusion in Iowa Waste Reduction Center Book Gallery by an authorized administrator of UNI ScholarWorks. For more information, please contact [email protected]. Manual2003 12/17/03 7:54 AM Page 2 © Copyright 2003 IOWA WASTE REDUCTION CENTER University of Northern Iowa Creation of this manual was funded by the U.S. Environmental Protection Agency, Risk Reduction Engineering Lab under Cooperative Agreement CR 821492-01-2. (Edition 1) The revision of this manual was funded by the U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics under a grant administered to the Small Business Pollution Prevention Center, Award Number X-82849601-3 (Edition 3) Cutting Fluid Management for Small Machining Operations Manual2003 12/17/03 7:54 AM Page 3 TABLE OF CONTENTS 1.0 INTRODUCTION -

Cutting and Grinding Oils Non Water-Miscible Coolants
Cutting and Grinding Oils Non water-miscible coolants BECHEM – Lubrication solutions for industry PICTOGRAMS APPLICATIONS As the oldest German manufacturer of industrial lubri- cants, BECHEM is one of the leading producers of Broaching high-quality special lubricants and metal working fluids Deep drilling today. Tool production BECHEM products convince through innovative for- mulations in the most varied industrial applications – in Automotive industry machining and forming metal working processes, in coating technology and as lifetime lubricants in various technical components. PROPERTIES A strong network of distributors and several national and international production sites ensure that BECHEM Extended tool life products are readily available worldwide. Chlorine-free Tomorrow’s technologies. Today. Low foaming Corrosion protection Good rinsing performance Minimum quantity lubrication Biodegradable 02 03 Maximum process reliability ALMOST OIL MIST FREE BASE OILS LUBRICATION ADDITIVES BECHEM process fluids have proven their reliability in various processes. Based on innovative additive com- ponents, these fluids guarantee reliable and economic machining of standard alloyed steel, high-tensile special materials and nickel alloys. The BECHEM lubricant sys- BASE FLUID tems allow production of the most demanding products DEFOAMER AW/EP ADDITIVES with excellent surface qualities from highly sensitive alu- minium alloys and lightweight construction materials. We put great emphasis on the compatibility of the BECHEM CORROSION PROTECTION products -

The Effect of Different Non-Metallic Inclusions on the Machinability of Steels
Materials 2015, 8, 751-783; doi:10.3390/ma8020751 OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Review The Effect of Different Non-Metallic Inclusions on the Machinability of Steels Niclas Ånmark 1,2,*, Andrey Karasev 1 and Pär Göran Jönsson 1 1 Department of Materials Science and Engineering, KTH Royal Institute of Technology, Stockholm SE-100-44, Sweden; E-Mails: [email protected] (A.K.); [email protected] (P.G.J.) 2 Department of Materials and Manufacturing, Swerea KIMAB, Kista SE-164-40, Sweden * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +46-0-72-508-43-42. Academic Editor: Richard Thackray Received: 19 December 2014 / Accepted: 11 February 2015 / Published: 16 February 2015 Abstract: Considerable research has been conducted over recent decades on the role of non-metallic inclusions and their link to the machinability of different steels. The present work reviews the mechanisms of steel fractures during different mechanical machining operations and the behavior of various non-metallic inclusions in a cutting zone. More specifically, the effects of composition, size, number and morphology of inclusions on machinability factors (such as cutting tool wear, power consumption, etc.) are discussed and summarized. Finally, some methods for modification of non-metallic inclusions in the liquid steel are considered to obtain a desired balance between mechanical properties and machinability of various steel grades. Keywords: inclusions; machinability; steel 1. Introduction Advances in steelmaking during the last six decades have resulted in steel grades with very low level of impurities. In recent years, new “clean and ultra-clean” steels have been developed and commercialized by steel producers around the world, thereby responding to the current and future market demands of steel having significantly improved mechanical properties (e.g., fatigue strength and impact toughness) and an improved corrosion resistance.