The Pyrenean Chamois Johan Espunyes Nozières
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
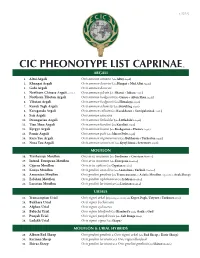
Cic Pheonotype List Caprinae©
v. 5.25.12 CIC PHEONOTYPE LIST CAPRINAE © ARGALI 1. Altai Argali Ovis ammon ammon (aka Altay Argali) 2. Khangai Argali Ovis ammon darwini (aka Hangai & Mid Altai Argali) 3. Gobi Argali Ovis ammon darwini 4. Northern Chinese Argali - extinct Ovis ammon jubata (aka Shansi & Jubata Argali) 5. Northern Tibetan Argali Ovis ammon hodgsonii (aka Gansu & Altun Shan Argali) 6. Tibetan Argali Ovis ammon hodgsonii (aka Himalaya Argali) 7. Kuruk Tagh Argali Ovis ammon adametzi (aka Kuruktag Argali) 8. Karaganda Argali Ovis ammon collium (aka Kazakhstan & Semipalatinsk Argali) 9. Sair Argali Ovis ammon sairensis 10. Dzungarian Argali Ovis ammon littledalei (aka Littledale’s Argali) 11. Tian Shan Argali Ovis ammon karelini (aka Karelini Argali) 12. Kyrgyz Argali Ovis ammon humei (aka Kashgarian & Hume’s Argali) 13. Pamir Argali Ovis ammon polii (aka Marco Polo Argali) 14. Kara Tau Argali Ovis ammon nigrimontana (aka Bukharan & Turkestan Argali) 15. Nura Tau Argali Ovis ammon severtzovi (aka Kyzyl Kum & Severtzov Argali) MOUFLON 16. Tyrrhenian Mouflon Ovis aries musimon (aka Sardinian & Corsican Mouflon) 17. Introd. European Mouflon Ovis aries musimon (aka European Mouflon) 18. Cyprus Mouflon Ovis aries ophion (aka Cyprian Mouflon) 19. Konya Mouflon Ovis gmelini anatolica (aka Anatolian & Turkish Mouflon) 20. Armenian Mouflon Ovis gmelini gmelinii (aka Transcaucasus or Asiatic Mouflon, regionally as Arak Sheep) 21. Esfahan Mouflon Ovis gmelini isphahanica (aka Isfahan Mouflon) 22. Larestan Mouflon Ovis gmelini laristanica (aka Laristan Mouflon) URIALS 23. Transcaspian Urial Ovis vignei arkal (Depending on locality aka Kopet Dagh, Ustyurt & Turkmen Urial) 24. Bukhara Urial Ovis vignei bocharensis 25. Afghan Urial Ovis vignei cycloceros 26. -

Low Sequence Diversity of the Prion Protein Gene (PRNP) in Wild Deer and Goat Species from Spain
Pitarch et al. Vet Res (2018) 49:33 https://doi.org/10.1186/s13567-018-0528-8 RESEARCH ARTICLE Open Access Low sequence diversity of the prion protein gene (PRNP) in wild deer and goat species from Spain José Luis Pitarch1, Helen Caroline Raksa1, María Cruz Arnal2, Miguel Revilla2, David Martínez2, Daniel Fernández de Luco2, Juan José Badiola1, Wilfred Goldmann3 and Cristina Acín1* Abstract The frst European cases of chronic wasting disease (CWD) in free-ranging reindeer and wild elk were confrmed in Norway in 2016 highlighting the urgent need to understand transmissible spongiform encephalopathies (TSEs) in the context of European deer species and the many individual populations throughout the European continent. The genetics of the prion protein gene (PRNP) are crucial in determining the relative susceptibility to TSEs. To establish PRNP gene sequence diversity for free-ranging ruminants in the Northeast of Spain, the open reading frame was sequenced in over 350 samples from fve species: Iberian red deer (Cervus elaphus hispanicus), roe deer (Capreolus capreolus), fallow deer (Dama dama), Iberian wild goat (Capra pyrenaica hispanica) and Pyrenean chamois (Rupicapra p. pyrenaica). Three single nucleotide polymorphisms (SNPs) were found in red deer: a silent mutation at codon 136, and amino acid changes T98A and Q226E. Pyrenean chamois revealed a silent SNP at codon 38 and an allele with a single octapeptide-repeat deletion. No polymorphisms were found in roe deer, fallow deer and Iberian wild goat. This appar- ently low variability of the PRNP coding region sequences of four major species in Spain resembles previous fndings for wild mammals, but implies that larger surveys will be necessary to fnd novel, low frequency PRNP gene alleles that may be utilized in CWD risk control. -

6.5 X 11 Double Line.P65
Cambridge University Press 978-0-521-76059-1 - Ungulate Management in Europe: Problems and Practices Edited by Rory Putman, Marco Apollonio and Reidar Andersen Index More information Index adaptive management 134–5, 180–2, 377–9 chronic wasting disease 203 Alces alces – see moose climate change Ammotragus lervia – see Barbary sheep effects on ungulate populations 349–66 Axis axis – see axis deer effects on disease and disease transmission 203, axis deer 13, 35, 36 320, 335, 337–40, 360–1 conservation 4, 19, 26, 30, 32, 33, 39–41, 380 Barbary sheep 32, 35, 36 cultural attitudes to hunting 4, 5–9 Bison bonasus – see bison, European bison, European 15, 27–9, 32, 33, 38, 40, 269–70, Dama dama – see fallow deer 275, 301, 353 damage and its management 144–82 blue tongue virus 195, 199, 205, 208, 333, 338–9 damage to agricultural crops 144, 151, 169–71 boar, European wild 15, 81, 89, 91, 92, 93, 94–5, damage to forestry 35, 144, 149–50, 151, 96, 99, 117, 126, 129, 144, 170–1, 175, 200, 171–3 288, 292–4, 300, 301, 303, 322, 327, 334, 335, damage to conservation habitats 13, 35, 37, 43, 336, 355, 365 144, 173–5 bovine tuberculosis 194, 196, 200–1, 208, 322, damage, compensation for 68, 70–1, 169, 171 327, 334 damage, control of 176 brucellosis 197, 202, 208, 327 deer–vehicle collisions – see ungulate–vehicle collisions Capra aegagrus – see goat, European wild disease 192–209, 319–37 Capra ibex – see ibex, alpine disease surveillance 130, 203–4, 328–30 Capra pyrenaica – see ibex, Spanish ungulates as vectors for diseases of livestock or Capreolus -

Temporal Variation in Foraging Activity and Grouping Patterns in a Mountain-Dwelling Herbivore Environmental and Endogenous
Behavioural Processes 167 (2019) 103909 Contents lists available at ScienceDirect Behavioural Processes journal homepage: www.elsevier.com/locate/behavproc Temporal variation in foraging activity and grouping patterns in a mountain-dwelling herbivore: Environmental and endogenous drivers T ⁎ Niccolò Fattorinia, , Claudia Brunettia, Carolina Baruzzia, Gianpasquale Chiatanteb, Sandro Lovaria,c, Francesco Ferrettia a Department of Life Sciences, University of Siena. Via P.A. Mattioli 4, 53100 Siena, Italy b Department of Earth and Environmental Sciences, University of Pavia, Via Ferrata 1, 27100 Pavia, Italy c Maremma Natural History Museum, Strada Corsini 5, 58100 Grosseto, Italy ARTICLE INFO ABSTRACT Keywords: In temperate ecosystems, seasonality influences animal behaviour. Food availability, weather, photoperiod and Seasonality endogenous factors relevant to the biological cycle of individuals have been shown as major drivers of temporal Foraging Activity changes in activity rhythms and group size/structure of herbivorous species. We evaluated how diurnal female Group size foraging activity and grouping patterns of a mountain herbivore, the Apennine chamois Rupicapra pyrenaica Rupicapra ornata, varied during a decreasing gradient of pasture availability along the summer-autumn progression Chamois (July–October), a crucial period for the life cycle of mountain ungulates. Mountain herbivores Females increased diurnal foraging activity, possibly because of constrains elicited by variation in environ- mental factors. Size of mixed groups did not vary, in contrast with the hypothesis that groups should be smaller when pasture availability is lower. Proportion of females in groups increased, possibly suggesting that they concentrated on patchily distributed nutritious forbs. Occurrence of yearlings in groups decreased, which may have depended on dispersal of chamois in this age class. -

Diversity and Evolution of the Mhc-DRB1 Gene in the Two Endemic Iberian Subspecies of Pyrenean Chamois, Rupicapra Pyrenaica
Heredity (2007) 99, 406–413 & 2007 Nature Publishing Group All rights reserved 0018-067X/07 $30.00 www.nature.com/hdy ORIGINAL ARTICLE Diversity and evolution of the Mhc-DRB1 gene in the two endemic Iberian subspecies of Pyrenean chamois, Rupicapra pyrenaica J Alvarez-Busto1, K Garcı´a-Etxebarria1, J Herrero2,3, I Garin4 and BM Jugo1 1Genetika, Antropologia Fisikoa eta Animali Fisiologia Saila, Zientzia eta Teknologia Fakultatea, Euskal Herriko Unibertsitatea (UPV/EHU), Bilbao, Spain; 2Departamento de Ecologı´a, Universidad de Alcala´ de Henares, Alcala´ de Henares, Spain; 3EGA Wildlife Consultants, Zaragoza, Spain and 4Zoologı´a eta Animali Zelulen Biologia Saila, Zientzia eta Teknologia Fakultatea, Euskal Herriko Unibertsitatea (UPV/EHU), Bilbao, Spain Major histocompatibility complex class II locus DRB variation recent parasitic infections by sarcoptic mange. A phyloge- was investigated by single-strand conformation polymorph- netic analysis of both Pyrenean chamois and DRB alleles ism analysis and sequence analysis in the two subspecies of from 10 different caprinid species revealed that the chamois Pyrenean chamois (Rupicapra pyrenaica) endemic to the alleles form two monophyletic groups. In comparison with Iberian Peninsula. Low levels of genetic variation were other Caprinae DRB sequences, the Rupicapra alleles detected in both subspecies, with seven different alleles in R. displayed a species-specific clustering that reflects a large p. pyrenaica and only three in the R. p. parva. After applying temporal divergence of the chamois from other caprinids, as the rarefaction method to cope with the differences in sample well as a possible difference in the selective environment for size, the low allele number of parva was highlighted. -

Infection Studies with Chamois Border Disease Virus in Pyrenean Chamois, Sheep and Pig
INFECTION STUDIES WITH CHAMOIS BORDER DISEASE VIRUS IN PYRENEAN CHAMOIS, SHEEP AND PIG ÒSCAR CABEZÓN PONSODA Directores: Ignasi Marco Sánchez Joaquim Segalés i Coma Departament de Medicina i Cirurgia Animals Facultat de Veterinària Universitat Autònoma de Barcelona 2011 1 2 Los Doctores Ignasi MARCO SÁNCHEZ y Joaquim SEGALÉS I COMA, Profesores Titulares de Universidad de las Áreas de conocimiento de Medicina y Cirugía Animal y Sanidad y Anatomía Animal, respectivamente, de la Facultad de Veterinaria de la Universitat Autònoma de Barcelona, HACEN CONSTAR, Que la memoria titulada “INFECTION STUDIES WITH CHAMOIS BORDER DISEASE VIRUS IN PYRENEAN CHAMOIS, SHEEP AND PIG”, presentada por Òscar Cabezón Ponsoda para la obtención del grado de Doctor en Veterinaria por la Universitat Autònoma de Barcelona, ha sido realizada bajo nuestra dirección y, considerándola satisfactoriamente finalizada, autorizamos su presentación para que sea juzgada por la comisión correspondiente. Y para que conste a los efectos oportunos, firmamos el presente informe en Bellaterra, a 1 de Junio de 2011. Firmado: Ignasi Marco Sánchez Firmado: Joaquim Segalés i Coma 3 4 AGRADECIMIENTOS No es habitual empezar los agradecimientos con alguien que no sea tu director de tesis, pero debo hacerlo. Y es que tengo que agradecer a Santiago Lavín primero, los 5 años que llevo en el SEFaS, y segundo, por la posibilidad de haber realizado esta tesis doctoral. Gracias, Santiago, por tener el convencimiento de que iba a ser así. Sin duda, y también antes de mis dos directores de Tesis, tengo que mencionar a Rosa Rosell. A Rosa le tengo que agradecer todo. Ella ha sido y es el mayor aliado que he tenido en todos los trabajos realizados. -

Brochure Highlight Those Impressive Russia
2019 44 years and counting The products and services listed Join us on Facebook, follow us on Instagram or visit our web site to become one Table of Contents in advertisements are offered and of our growing number of friends who receive regular email updates on conditions Alaska . 4 provided solely by the advertiser. and special big game hunt bargains. Australia . 38 www.facebook.com/NealAndBrownleeLLC Neal and Brownlee, L.L.C. offers Austria . 35 Instagram: @NealAndBrownleeLLC no guarantees, warranties or Azerbaijan . 31 recommendations for the services or Benin . 18 products offered. If you have questions Cameroon . 19 related to these services, please contact Canada . 6 the advertiser. Congo . 20 All prices, terms and conditions Continental U .S . 12 are, to the best of our knowledge at the Ethiopia . 20 time of printing, the most recent and Fishing Alaska . 42 accurate. Prices, terms and conditions Fishing British Columbia . 41 are subject to change without notice Fishing New Zealand . 42 due to circumstances beyond our Kyrgyzstan . 31 control. Jeff C. Neal Greg Brownlee Trey Sperring Mexico . 14 Adventure travel and big game 2018 was another fantastic year for our company thanks to the outfitters we epresentr and the Mongolia . 32 hunting contain inherent risks and clients who trusted us. We saw more clients traveling last season than in any season in the past, Mozambique . 21 dangers by their very nature that with outstanding results across the globe. African hunting remained strong, with our primary Namibia . 22 are beyond the control of Neal and areas producing outstanding success across several countries. Asian hunting has continued to be Nepal . -

Auction Preview
AUCTION PREVIEW Online: TO DONATE CONTACT: KIM NIETERS | 406.404.8764 | [email protected] A1 3 - DAY/4-NIGHT FISHING A1 A3 TRIP FOR 2 WITH RAWHIDE OUTFITTERS IN IDAHO (100% FULLY DONATED) RAWHIDE OUTFITTERS Cathy or Luke Cranney P: 208.756.4276 E: [email protected] W: www.rawhideoutfitters.com A2 A2 “BOBCAT NAP” FRAMED PHOTOGRAPH ON ALUMINUM (100% FULLY DONATED) GREAT WILDLIFE PHOTOS Lynn Starnes P: 775.384.3430 E: [email protected] W: www.greatwildlifephotos.com A4 A3 2 HUNTERS FOR SAAM™ PRECISION & SAFARI HUNT COMBO AND $2,000 TOWARD TROPHY FEES FOR EACH HUNTER Tim Fallon FTW RANCH P: 830.234.4366 or 864.814.3220 E: [email protected] A4 7 - DAY HUNT IN SOUTH AFRICA FOR 2 HUNTERS W/$1000 TROPHY FEE CREDIT PER HUNTER & $500 CREDIT TOWARDS A PHOTO SAFARIS (100% FULLY DONATED) A5 IBAMBA SAFARIS Johan Pretorius E: [email protected] W: www.ibambasafaris.com A5 7 - DAY CROCODILE HUNT IN MPUMALONGA SOUTH AFRICA FOR 1 HUNTER AND 1 NON-HUNTER (Trophy Fee is Included) ROCHE SAFARIS Roche du Preez P: 27.836.765.748 E: [email protected] W: www.rochesafaris.com A6 A6 SPRINGFIELD ARMORY M1A RIFLE 308 WIN FRIENDS OF WSF P: 406.404.8750 A7 NEW ZEALAND 10-DAY RED STAG, BULL TAHR, ALPINE A7 CHAMOIS HUNT FOR 1 to 6 HUNTERS (One red stag trophy fee included up to 379 SCI per hunter. Trophy fees for bull tahr, Alpine chamois are the responsibility of the buyers ) NEW ZEALAND HORN & ANTLER SAFARIS Craig Dempster P: +64.3.689.1377 E: [email protected] W: www.hornandantler.com A8 1 - YEAR SUBSCRIPTION NOVEMBER-DECEMBER -

Molecular Identification of a New Pestivirus Associated With
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Diposit Digital de Documents de la UAB Journal of Wildlife Diseases, 40(4), 2004, pp. 796±800 q Wildlife Disease Association 2004 Molecular Identi®cation of a New Pestivirus Associated with Increased Mortality in the Pyrenean Chamois (Rupicapra pyrenaica pyrenaica) in Spain Ana Hurtado,1,3 Gorka Aduriz,1 Nieves Go mez,1 Beatriz Oporto,1 Ramo n A. Juste,1 Santiago Lavin,2 Jorge R. Lopez-Olvera,2 and Ignasi Marco21Department of Animal Health, Instituto Vasco de InvestigacioÂn y De- sarrollo Agrario Neiker, Berreaga 1, 48160 Derio, Bizkaia, Spain; 2 Servei d'Ecopatologia de Fauna Salvatge, Facultat de Veterinaria, Universitat Autonoma de Barcelona, 08193-Bellaterra, Spain; 3 Corresponding author (email: [email protected]) ABSTRACT: Pestivirus infection was identi®ed stock can cause a variety of economically in 16 of 17 chamois during an outbreak of a important syndromes (Houe, 1999), in- previously unreported disease in Pyrenean chamois (Rupicapra pyrenaica pyrenaica)in cluding reproductive failure, poor devel- northeastern Spain in 2001±02. By analysis of opment, enteric disease, and a hemorrhag- the 59 noncoding regions of the virus, we as- ic syndrome. Fetal infection can lead to signed it to the border disease virus cluster with birth of persistently infected (PI) animals pairwise similarity values ranging from 82.1% to 88.1%. It will be important to investigate the that become chronic shedders of the virus. association of this pestivirus with disease in In goats, natural infections with pestivirus- Pyrenean chamois. es do not appear to be a problem because Key words: Border disease virus, chamois, ®eld cases of clinical disease are rare. -

The U.K. Hunter Who Has Shot More Wildlife Than the Killer of Cecil the Lion
CAMPAIGN TO BAN TROPHY HUNTING Special Report The U.K. hunter who has shot more wildlife than the killer of Cecil the Lion SUMMARY The Campaign to Ban Trophy Hunting is revealing the identity of a British man who has killed wild animals in 5 continents, and is considered to be among the world’s ‘elite’ in the global trophy hunting industry. Malcolm W King has won a staggering 36 top awards with Safari Club International (SCI), and has at least 125 entries in SCI’s Records Book. The combined number of animals required for the awards won by King is 528. Among his awards are prizes for shooting African ‘Big Game’, wild cats, and bears. King has also shot wild sheep, goats, deer and oxen around the world. His exploits have taken him to Asia, Africa and the South Pacific, as well as across Europe. The Campaign to Ban Trophy Hunting estimates that around 1.7 million animals have been killed by trophy hunters over the past decade, of which over 200,000 were endangered species. Lions are among those species that could be pushed to extinction by trophy hunting. An estimated 10,000 lions have been killed by ‘recreational’ hunters in the last decade. Latest estimates for the African lion population put numbers at around 20,000, with some saying they could be as low as 13,000. Industry groups like Safari Club International promote prizes which actively encourage hunters to kill huge numbers of endangered animals. The Campaign to Ban Trophy Hunting believes that trophy hunting is an aberration in a civilised society. -

Rarity, Trophy Hunting and Ungulates L
Animal Conservation. Print ISSN 1367-9430 Rarity, trophy hunting and ungulates L. Palazy1,2, C. Bonenfant1, J. M. Gaillard1 & F. Courchamp2 1 Biometrie´ et Biologie E´ volutive, Villeurbanne, France 2 Ecologie, Systematique´ et Evolution, Orsay, France Keywords Abstract anthropogenic Allee effect; conservation; hunting management; trophy price; The size and shape of a trophy constitute major determinants of its value. mammals. We postulate that the rarity of a species, whatever its causes, also plays a major role in determining its value among hunters. We investigated a role for an Correspondence Anthropogenic Allee effect in trophy hunting, where human attraction to rarity Lucille Palazy. Current address: UMR CNRS could lead to an over-exploitative chain reaction that could eventually drive the 5558, Univ Lyon 1, 43 bd 11 nov, 69622 targeted species to extinction. We performed an inter-specific analysis of trophy Villeurbanne cedex, France. prices of 202 ungulate taxa and quantified to what extent morphological char- Tel: +33 4.72.43.29.35 acteristics and their rarity accounted for the observed variation in their price. We Email: [email protected] found that once location and body mass were accounted for, trophies of rare species attain higher prices than those of more common species. By driving trophy Editor: Iain Gordon price increase, this rarity effect may encourage the exploitation of rare species Associate Editor: John Linnell regardless of their availability, with potentially profound consequences for populations. Received 24 January 2011; accepted 16 May 2011 doi:10.1111/j.1469-1795.2011.00476.x Introduction et al., 1997). Moreover, the current worldwide trend of increasing wealth, in particular in the Middle East, Russia Over-exploitation of natural resources by humans is one and China (Dubois & Laurent, 1998; Guriev & Rachinsky, of the main causes of the current and dramatic loss of 2009), is likely to be paralleled by a growth in demand for biodiversity (Kerr & Currie, 1995; Burney & Flannery, sport hunting. -

Inter-Agency Coordination Fosters the Recovery of the Pyrenean Chamois Rupicapra Pyrenaica Pyrenaica at Its Western Limit
Short Communication Inter-agency coordination fosters the recovery of the Pyrenean chamois Rupicapra pyrenaica pyrenaica at its western limit J uan H errero,Inazio G arin,Carlos P rada and A licia G arc´I A -SERRANO Abstract Two political jurisdictions in northern Spain, overlap jurisdictions, coordinated management is relatively Navarre, where the Pyrenean chamois Rupicapra pyrenaica common (Proctor et al., 2004; Antor et al., 2007). pyrenaica is categorized as Vulnerable, and Aragon, where Ungulates have life histories that are conducive to it is huntable, coordinated management for the recovery of sustainable harvests (Caughley & Sinclair, 1994) and, for this subspecies at the western limit of its range. After an a variety of reasons (population control, meat production, estimate of only a small population in 1992–1993 hunting and recreational and trophy hunting), are intensively was banned in Aragon and, in 1995–1996, monitoring of exploited. In addition, threatened populations have re- both populations began. In the two massifs where the covered (e.g. Arabian oryx Oryx leucoryx, IUCN SSC subspecies lives, which lie across the border of the two Antelope Specialist Group, 2008) and conservation efforts jurisdictions, chamois populations increased from 33 to can have a positive effect (e.g. bison Bison bonasus, Pucek, 136 and from 144 to 455 by 2007 (average annual increases 2004; Apennine chamois Rupicapra pyrenaica ornata, were 15 and 11%, respectively). The subspecies was also Dupre´ et al., 2001) although, in some cases, conservation located on a third massif, in 2002. After the recovery the efforts have been initiated too late (e.g. Pyrenean wild goat ban against hunting was lifted in Aragon, in 2006, with Capra pyrenaica; Garcı´a-Gonza´lez & Herrero, 1999).