Long-Term Outcomes and Practical Considerations in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -
Second-Tier DNA Confirmation of Newborn Screening Results
Second-tier DNA Confirmation of Newborn Screening by Targeted Next Generation Sequencing Edwin Naylor, Ph.D. MPH Andy Bhattacharjee , Ph.D. Erik Puffenberger, Ph.D.; Kevin Strauss, MD; Holmes Morton, MD Newborn Screening & Clinical Genomics 1961 1990’s 2010-2012 2 Robert Guthrie Development of develops simple automated MS/MS Newborn Screening screening across (NBS) several disorders Current de facto standard 2 Why Newborn Genomics? • Mendelian Diseases disproportionately affect Newborns - ~3500 genetic diseases with molecular basis - >10% of NICU admissions are genetic Clinical manifestation of Genetic diseases - Current NBS tests limited to 29+ diseases CHROMOSOMAL - 2nd tier DNA testing to validate biochemical results MULTI-FACTORIAL SINGLE GENE (MENDELIAN) • Advantage of NGS based DNA testing individuals # of Affected - Find causal variants (rare/novel) in gene(s) - A ‘universal’ NGS approach avoids repeated, serial BIRTH PUBERTY ADULT single gene testing Gelehrter TD, Collins FS, Ginsburg D. Principles of - Current Sanger sequencing is expensive ($3-10K) and Medical Genetics. 2nd ed. Baltimore, MD: Williams & slow (3 months to 1 year) Wilkins; 1998:1-42 NICU- Neonatal Intensive Care Unit NBS-Newborn Screening 3 NGS-Next Generation Sequencing Why Targeted (Exome) Sequencing for now? NGS Sequencing * Genomic DNA from Causal Mutations in Affected Individuals Exons/Target Regions Fold Test Menu Cost ($) Throughput Efficiency Whole Genome (Res.) 7,666* 1 1 Exome (Res) 1,200 15 95 Neonate Panel (Clinical) <1000 150 >1140 •Majority of known disease-causing mutations in exons •Exome = protein-encoding parts of genes •Targeted NGS is Cost & Throughput Efficient *Saunders et al., (2012) Rapid Whole Genome Sequencing for Genetic Disease Diagnosis in NICUs 4 Workflow for 2nd Tier Newborn Screening Sample 2h DNA Capture 92h Raw Data 10h Analysis 1h+ Isolation & Sequencing Management & Interpretation 8 samples, 105 Hrs, <$10,000 = Real Neonatal Genomics! 5 Workflow for 2nd Tier Newborn Screening Sample •High M.Wt. -

Newborn Screening Laboratory Manual of Services
Newborn Screening Laboratory Manual of Services Test Panel: Please see the following links for a detailed description of testing in the Newborn Screening section. Information about the Newborn Screening program is available here. Endocrine Disorders Congenital adrenal hyperplasia (CAH) Congenital hypothyroidism (TSH) Hemoglobinopathies Sickle cell disease (FS) Alpha (Barts) Sickle βeta Thalassemia (FSA) Other sickling hemoglobinopathies such as: FAS FAC FAD FAE Homozygous conditions such as: FC FD FE Metabolic Disorders Biotinidase deficiency Galactosemia Cystic fibrosis (CF) first tier screening for elevated immunoreactive trypsinogen (IRT) Cystic fibrosis second tier genetic mutation analysis on the top 4% IRT concentrations. Current alleles detected : F508del, I507del, G542X, G85E, R117H, 621+1G->T, 711+1G->T, R334W, R347P, A455E, 1717-1G->A, R560T, R553X, G551D, 1898+1G->A, 2184delA, 2789+5G->A, 3120+1G->A, R1162X, 3659delC, 3849+10kbC->T, W1282X, N1303K, IVS polyT T5/T7/T9 *Currently validating a mutation panel that includes the above alleles in addition to the following: 1078delT, Y122X, 394delTT, R347H, M1101K, S1255X, 1898+5G->T, 2183AA->G, 2307insA, Y1092X, 3876delA, 3905insT, S549N, S549R_1645A->C, S549R-1647T->G, S549R-1647T->G, V520F, A559T, 1677delTA, 2055del9->A, 2143delT, 3199del6, 406-1G->A, 935delA, D1152H, CFTRdele2, E60X, G178R, G330X, K710X, L206W, Q493X, Q890X, R1066C, R1158X, R75X, S1196X, W1089X, G1244E, G1349D, G551S, R560KT, S1251N, S1255P Amino acid disorders Phenylketonuria (PKU) / Hyperphenylalaninemia Maple -

University of Cincinnati
UNIVERSITY OF CINCINNATI Date: August 29, 2006 I, Smita Chawla hereby submit this work as a part of the requirements of the degree of: Doctor of Philosophy (Ph. D.) in: Pharmaceutical Sciences It is entitled: Effect of deoxyArbutin and it’s second-generation derivatives on melanocyte viability and function. on Human Nail Permeabili ty . This work and its defense approved by: R. Randall Wickett, Ph.D., Chair Raymond E. Boissy, Ph.D. William Cacini, Ph.D. Prashiela Manga, Ph.D. Marty O. Visscher, Ph.D. EFFECT OF DEOXYARBUTIN AND ITS SECOND-GENERATION DERIVATIVES ON MELANOCYTE VIABILITY AND FUNCTION A dissertation submitted to the Division of Research and Advanced Studies of the University of Cincinnati in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in the Division of Pharmaceutical Sciences of the College of Pharmacy 2006 by Smita Chawla B.S. in Pharmaceutical Sciences Mumbai University, Mumbai, India, 2001 Committee Chair: Randall Wickett, Ph.D. ABSTRACT Therapeutic treatment of skin pigmentary disorders such as melasma, solar lentigines, and post inflammatory hyperpigmentation has been challenging and seldom completely successful. The majority of these therapies target tyrosinase, a key enzyme required for pigment synthesis. The lack of success is due to the less than desired efficacy and safety of tyrosinase inhibitors marketed as skin lightening agents (i.e. hydroquinone, kojic acid and arbutin). We propose that the tyrosinase inhibitor deoxyArbutin (dA) and second-generation derivatives of dA, deoxyFuran, thio-dA, and fluoro-dA, have the potential to be effective inhibitors of skin melanization. In this study, we have analyzed the modulating effects of dA and its derivatives on melanocyte function and viability. -

Inherited Metabolic Disease
Inherited metabolic disease Dr Neil W Hopper SRH Areas for discussion • Introduction to IEMs • Presentation • Initial treatment and investigation of IEMs • Hypoglycaemia • Hyperammonaemia • Other presentations • Management of intercurrent illness • Chronic management Inherited Metabolic Diseases • Result from a block to an essential pathway in the body's metabolism. • Huge number of conditions • All rare – very rare (except for one – 1:500) • Presentation can be non-specific so index of suspicion important • Mostly AR inheritance – ask about consanguinity Incidence (W. Midlands) • Amino acid disorders (excluding phenylketonuria) — 18.7 per 100,000 • Phenylketonuria — 8.1 per 100,000 • Organic acidemias — 12.6 per 100,000 • Urea cycle diseases — 4.5 per 100,000 • Glycogen storage diseases — 6.8 per 100,000 • Lysosomal storage diseases — 19.3 per 100,000 • Peroxisomal disorders — 7.4 per 100,000 • Mitochondrial diseases — 20.3 per 100,000 Pathophysiological classification • Disorders that result in toxic accumulation – Disorders of protein metabolism (eg, amino acidopathies, organic acidopathies, urea cycle defects) – Disorders of carbohydrate intolerance – Lysosomal storage disorders • Disorders of energy production, utilization – Fatty acid oxidation defects – Disorders of carbohydrate utilization, production (ie, glycogen storage disorders, disorders of gluconeogenesis and glycogenolysis) – Mitochondrial disorders – Peroxisomal disorders IMD presentations • ? IMD presentations • Screening – MCAD, PKU • Progressive unexplained neonatal -

TYR I, II, III Act Sheet
Newborn Screening ACT Sheet Increased Tyrosine Tyrosinemia Differential Diagnosis: Tyrosinemia I (hepatorenal); Tyrosinemia II (oculocutaneous); Tyrosinemia III; transient hypertyrosinemia; liver disease. Condition Description: In the hepatorenal form, tyrosine from ingested protein and phenylalanine metabolism cannot be metabolized by fumarylacetoacetate hydrolase to fumaric acid and acetoacetic acid. The resulting fumarylacetoacetate accumulates and is converted to succinylacetone, the diagnostic metabolite, which is liver toxic, and leads to elevated tyrosine. Tyrosinemias II and III are due to other defects in tyrosine degradation. You Should Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result. • Consult with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn and refer as appropriate. • Initiate confirmatory/diagnostic tests in consultation with metabolic specialist. • Initial testing: plasma quantitative amino acids; urine succinylacetone and liver function tests. • Repeat newborn screen if the second screen has not been done. • Provide family with basic information about tyrosinemia. • Report findings to newborn screening program. Diagnostic Evaluation: Plasma quantitative amino acid analysis will show increased tyrosine in all of the tyrosinemias. Urine organic acid analysis may reveal increased succinylacetone in Tyrosinemia I. Clinical Considerations: Tyrosinemia I is usually asymptomatic in the neonate. If untreated, it will cause liver disease and cirrhosis -
Download This PDF File
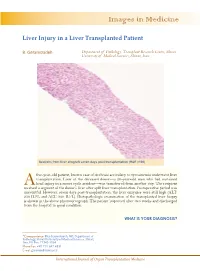
Images in Medicine Liver Injury in a Liver Transplanted Patient B. Geramizadeh Department of Pathology, Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Sections from liver allograft seven days post-transplantation (H&E ×100) five-year-old patient, known case of cirrhosis secondary to tyrosinemia underwent liver transplantation. Liver of the deceased donor—a 20-year-old man who had sustained A head injury in a motor cycle accident—was transferred from another city. The recipient received a segment of the donor’s liver after split liver transplantation. Postoperative period was uneventful. However, seven days post-transplantation, the liver enzymes were still high (ALT: 250 IU/L and ALT: 320 IU/L). Histopathologic examination of the transplanted liver biopsy is shown in the above photomicrograph. The patient improved after two weeks and discharged from the hospital in good condition. WHAT IS YOUR DIAGNOSIS? *Correspondence: Bita Geramizadeh, MD, Department of Pathology, Shiraz University of Medical Sciences, Shiraz, Iran. PO Box: 71345-1864 Phone/Fax: +98-711-647-4331 E-mail: [email protected] International Journal of Organ Transplantation Medicine B. Geramizadeh DIAGNOSIS: PRESERVATION/REPERFUSION INJURY dvances in organ preservation have duct obstruction. The distinctive pattern of reduced preservation injury. Neverthe- bile ductular cholestasis is that it is usually as- Aless, when storage time exceeds 10 to sociated with sepsis. Drug toxicity can mimic 12 hours, post-transplantation complications, every change in the liver and should always be due to preservation/reperfusion injury, be- excluded [5]. come more common [1]. In our patient, prolonged cold ischemic time Ischemic injury to the graft is divided into (transfer of the liver from another city) and cold ischemia—secondary to prolonged pres- probably small for size graft (split liver trans- ervation—and warm ischemia, which occurs plantation) were predisposing factors. -

What Disorders Are Screened for by the Newborn Screen?
What disorders are screened for by the newborn screen? Endocrine Disorders The endocrine system is important to regulate the hormones in our bodies. Hormones are special signals sent to various parts of the body. They control many things such as growth and development. The goal of newborn screening is to identify these babies early so that treatment can be started to keep them healthy. To learn more about these specific disorders please click on the name of the disorder below: English: Congenital Adrenal Hyperplasia Esapnol Hiperplasia Suprarrenal Congenital - - http://www.newbornscreening.info/Parents/otherdisorders/CAH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CAH.html - Congenital Hypothyroidism (Hipotiroidismo Congénito) - http://www.newbornscreening.info/Parents/otherdisorders/CH.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/CH.html Hematologic Conditions Hemoglobin is a special part of our red blood cells. It is important for carrying oxygen to the parts of the body where it is needed. When people have problems with their hemoglobin they can have intense pain, and they often get sick more than other children. Over time, the lack of oxygen to the body can cause damage to the organs. The goal of newborn screening is to identify babies with these conditions so that they can get early treatment to help keep them healthy. To learn more about these specific disorders click here (XXX). - Sickle Cell Anemia (Anemia de Célula Falciforme) - http://www.newbornscreening.info/Parents/otherdisorders/SCD.html - http://www.newbornscreening.info/spanish/parent/Other_disorder/SCD.html - SC Disease (See Previous Link) - Sickle Beta Thalassemia (See Previous Link) Enzyme Deficiencies Enzymes are special proteins in our body that allow for chemical reactions to take place. -

Amino Acid Disorders
471 Review Article on Inborn Errors of Metabolism Page 1 of 10 Amino acid disorders Ermal Aliu1, Shibani Kanungo2, Georgianne L. Arnold1 1Children’s Hospital of Pittsburgh, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; 2Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, MI, USA Contributions: (I) Conception and design: S Kanungo, GL Arnold; (II) Administrative support: S Kanungo; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: E Aliu, GL Arnold; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Georgianne L. Arnold, MD. UPMC Children’s Hospital of Pittsburgh, 4401 Penn Avenue, Suite 1200, Pittsburgh, PA 15224, USA. Email: [email protected]. Abstract: Amino acids serve as key building blocks and as an energy source for cell repair, survival, regeneration and growth. Each amino acid has an amino group, a carboxylic acid, and a unique carbon structure. Human utilize 21 different amino acids; most of these can be synthesized endogenously, but 9 are “essential” in that they must be ingested in the diet. In addition to their role as building blocks of protein, amino acids are key energy source (ketogenic, glucogenic or both), are building blocks of Kreb’s (aka TCA) cycle intermediates and other metabolites, and recycled as needed. A metabolic defect in the metabolism of tyrosine (homogentisic acid oxidase deficiency) historically defined Archibald Garrod as key architect in linking biochemistry, genetics and medicine and creation of the term ‘Inborn Error of Metabolism’ (IEM). The key concept of a single gene defect leading to a single enzyme dysfunction, leading to “intoxication” with a precursor in the metabolic pathway was vital to linking genetics and metabolic disorders and developing screening and treatment approaches as described in other chapters in this issue. -

Centometabolic® COMBINING GENETIC and BIOCHEMICAL TESTING for the FAST and COMPREHENSIVE DIAGNOSTIC of METABOLIC DISORDERS
CentoMetabolic® COMBINING GENETIC AND BIOCHEMICAL TESTING FOR THE FAST AND COMPREHENSIVE DIAGNOSTIC OF METABOLIC DISORDERS GENE ASSOCIATED DISEASE(S) ABCA1 HDL deficiency, familial, 1; Tangier disease ABCB4 Cholestasis, progressive familial intrahepatic 3 ABCC2 Dubin-Johnson syndrome ABCD1 Adrenoleukodystrophy; Adrenomyeloneuropathy, adult ABCD4 Methylmalonic aciduria and homocystinuria, cblJ type ABCG5 Sitosterolemia 2 ABCG8 Sitosterolemia 1 ACAT1 Alpha-methylacetoacetic aciduria ADA Adenosine deaminase deficiency AGA Aspartylglucosaminuria AGL Glycogen storage disease IIIa; Glycogen storage disease IIIb AGPS Rhizomelic chondrodysplasia punctata, type 3 AGXT Hyperoxaluria, primary, type 1 ALAD Porphyria, acute hepatic ALAS2 Anemia, sideroblastic, 1; Protoporphyria, erythropoietic, X-linked ALDH4A1 Hyperprolinemia, type II ALDOA Glycogen storage disease XII ALDOB Fructose intolerance, hereditary ALG3 Congenital disorder of glycosylation, type Id ALPL Hypophosphatasia, adult; Hypophosphatasia, childhood; Hypophosphatasia, infantile; Odontohypophosphatasia ANTXR2 Hyaline fibromatosis syndrome APOA2 Hypercholesterolemia, familial, modifier of APOA5 Hyperchylomicronemia, late-onset APOB Hypercholesterolemia, familial, 2 APOC2 Hyperlipoproteinemia, type Ib APOE Sea-blue histiocyte disease; Hyperlipoproteinemia, type III ARG1 Argininemia ARSA Metachromatic leukodystrophy ARSB Mucopolysaccharidosis type VI (Maroteaux-Lamy) 1 GENE ASSOCIATED DISEASE(S) ASAH1 Farber lipogranulomatosis; Spinal muscular atrophy with progressive myoclonic epilepsy -

Ex Vivo Gene Therapy: a “Cultured” Surgical Approach to Curing Inherited Liver Disease
Mini Review Open Access J Surg Volume 10 Issue 3 - March 2019 Copyright © All rights are reserved by Joseph B Lillegard DOI: 10.19080/OAJS.2019.10.555788 Ex Vivo Gene Therapy: A “Cultured” Surgical Approach to Curing Inherited Liver Disease Caitlin J VanLith1, Robert A Kaiser1,2, Clara T Nicolas1 and Joseph B Lillegard1,2,3* 1Department of Surgery, Mayo Clinic, Rochester, MN, USA 2Midwest Fetal Care Center, Children’s Hospital of Minnesota, Minneapolis, MN, USA 3Pediatric Surgical Associates, Minneapolis, MN, USA Received: February 22, 2019; Published: March 21, 2019 *Corresponding author: Joseph B Lillegard, Midwest Fetal Care Center, Children’s Hospital of Minnesota, Minneapolis, Minnesota, USA and Mayo Clinic, Rochester, Minnesota, USA Introduction Inborn errors of metabolism (IEMs) are a group of inherited diseases caused by mutations in a single gene [1], many of which transplant remains the only curative option. Between 1988 and 2018, 12.8% of 17,009 pediatric liver transplants in the United States(see were primarily due to an inherited liver). disease. are identified in Table 1. Though individually rare, combined incidence is about 1 in 1,000 live births [2]. While maintenance www.optn.transplant.hrsa.gov/data/ Table 1: List of 35 of the most common Inborn Errors of Metabolism. therapies exist for some of these liver-related diseases, Inborn Error of Metabolism Abbreviation Hereditary Tyrosinemia type 1 HT1 Wilson Disease Wilson Glycogen Storage Disease 1 GSD1 Carnitine Palmitoyl Transferase Deficiency Type 2 CPT2 Glycogen Storage -

Quality of Life and Photodermatoses in People with Albinism in Benin City Nigeria
QUALITY OF LIFE AND PHOTODERMATOSES IN PEOPLE WITH ALBINISM IN BENIN CITY NIGERIA DISSERTATION SUBMITTED TO NATIONAL POSTGRADUATE MEDICAL COLLEGE OF NIGERIA IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE FELLOWSHIP IN INTERNAL MEDICINE (SUB-SPECIALTY: DERMATOLOGY) BY DR CYNTHIA ROLI MADUBUKO MB,BS (BENIN) DEPARTMENT OF MEDICINE UNIVERSITY OF BENIN TEACHING HOSPITAL, BENIN CITY, EDO STATE, NIGERIA NOVEMBER, 2016. i DECLARATION I hereby declare that this work is original and no part of it has been presented to any other college for a fellowship dissertation nor has it been submitted elsewhere for publication. Signature.................................... Date........................... Dr. Cynthia Roli Madubuko ii SUPERVISION We the undersigned have supervised the writing of this dissertation and the execution of this study. SIGNATURE: …………………………………… DATE: …………………………………………… YEAR OF FELLOWSHIP: ……………………… DR. B. OKWARA MBBS, FMCP Consultant Physician/ Dermatologist and Venereologist Department of Internal Medicine, University of Benin Teaching Hospital, Benin City, Nigeria. SIGNATURE: …………………………………… DATE: …………………………………………… YEAR OF FELLOWSHIP: ……………………… PROF. A.N. ONUNU MBBS, FWACP, FACP Consultant Physician/ Dermatologist and Venereologist Department of Internal Medicine, University of Benin Teaching Hospital, Benin City, Nigeria. SIGNATURE: …………………………………… DATE: …………………………………………… YEAR OF FELLOWSHIP: ……………………… PROF. E.P KUBEYINJE MBBS, FRCP (London), FWACP Consultant Physician Dermatologist and Venereologist Department of Internal Medicine, University of Benin Teaching Hospital, Benin City, Nigeria. iii CERTIFICATION I certify that this is a part 2 dissertation of the National postgraduate Medical College of Nigeria by Dr. Cynthia Roli Madubuko, of the Department of Internal Medicine, University of Benin Teaching Hospital, Benin City, under the supervision of Prof. A.N Onunu, Prof. E.P. Kubeyinje and Dr. B. Okwara Sign……………………………………. Date.............................................. DR OMUEMU, MBBS, FWACP.