Intraspecific Morphological Root Trait Variation Is Less Affected by Soil Fertility Than Biomass Allocation Or Root Nutrient Concentrations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NEWSLETTER NUMBER 84 JUNE 2006 New Zealand Botanical Society
NEW ZEALAND BOTANICAL SOCIETY NEWSLETTER NUMBER 84 JUNE 2006 New Zealand Botanical Society President: Anthony Wright Secretary/Treasurer: Ewen Cameron Committee: Bruce Clarkson, Colin Webb, Carol West Address: c/- Canterbury Museum Rolleston Avenue CHRISTCHURCH 8001 Subscriptions The 2006 ordinary and institutional subscriptions are $25 (reduced to $18 if paid by the due date on the subscription invoice). The 2006 student subscription, available to full-time students, is $9 (reduced to $7 if paid by the due date on the subscription invoice). Back issues of the Newsletter are available at $2.50 each from Number 1 (August 1985) to Number 46 (December 1996), $3.00 each from Number 47 (March 1997) to Number 50 (December 1997), and $3.75 each from Number 51 (March 1998) onwards. Since 1986 the Newsletter has appeared quarterly in March, June, September and December. New subscriptions are always welcome and these, together with back issue orders, should be sent to the Secretary/Treasurer (address above). Subscriptions are due by 28th February each year for that calendar year. Existing subscribers are sent an invoice with the December Newsletter for the next years subscription which offers a reduction if this is paid by the due date. If you are in arrears with your subscription a reminder notice comes attached to each issue of the Newsletter. Deadline for next issue The deadline for the September 2006 issue is 25 August 2006 Please post contributions to: Joy Talbot 17 Ford Road Christchurch 8002 Send email contributions to [email protected] or [email protected]. Files are preferably in MS Word (Word XP or earlier) or saved as RTF or ASCII. -

The New Zealand Rain Forest: a Comparison with Tropical Rain Forest! J
The New Zealand Rain Forest: A Comparison with Tropical Rain Forest! J. W. DAWSON2 and B. V. SNEDDON2 ABSTRACT: The structure of and growth forms and habits exhibited by the New Zealand rain forest are described and compared with those of lowland tropical rain forest. Theories relating to the frequent regeneration failure of the forest dominants are outlined. The floristic affinities of the forest type are discussed and it is suggested that two main elements can be recognized-lowland tropical and montane tropical. It is concluded that the New Zealand rain forest is comparable to lowland tropical rain forest in structure and in range of special growth forms and habits. It chiefly differs in its lower stature, fewer species, and smaller leaves. The floristic similarity between the present forest and forest floras of the Tertiary in New Zealand suggest that the former may be a floristically reduced derivative of the latter. PART 1 OF THIS PAPER describes the structure The approximate number of species of seed and growth forms of the New Zealand rain plants in these forests is 240. From north to forest as exemplified by a forest in the far north. south there is an overall decrease in number of In Part 2, theories relating to the regeneration species. At about 38°S a number of species, of the dominant trees in the New Zealand rain mostly trees and shrubs, drop out or become forest generally are reviewed briefly, and their restricted to coastal sites, but it is not until about relevance to the situation in the study forest is 42°S, in the South Island, that many of the con considered. -

The Correspondence of Julius Haast and Joseph Dalton Hooker, 1861-1886
The Correspondence of Julius Haast and Joseph Dalton Hooker, 1861-1886 Sascha Nolden, Simon Nathan & Esme Mildenhall Geoscience Society of New Zealand miscellaneous publication 133H November 2013 Published by the Geoscience Society of New Zealand Inc, 2013 Information on the Society and its publications is given at www.gsnz.org.nz © Copyright Simon Nathan & Sascha Nolden, 2013 Geoscience Society of New Zealand miscellaneous publication 133H ISBN 978-1-877480-29-4 ISSN 2230-4495 (Online) ISSN 2230-4487 (Print) We gratefully acknowledge financial assistance from the Brian Mason Scientific and Technical Trust which has provided financial support for this project. This document is available as a PDF file that can be downloaded from the Geoscience Society website at: http://www.gsnz.org.nz/information/misc-series-i-49.html Bibliographic Reference Nolden, S.; Nathan, S.; Mildenhall, E. 2013: The Correspondence of Julius Haast and Joseph Dalton Hooker, 1861-1886. Geoscience Society of New Zealand miscellaneous publication 133H. 219 pages. The Correspondence of Julius Haast and Joseph Dalton Hooker, 1861-1886 CONTENTS Introduction 3 The Sumner Cave controversy Sources of the Haast-Hooker correspondence Transcription and presentation of the letters Acknowledgements References Calendar of Letters 8 Transcriptions of the Haast-Hooker letters 12 Appendix 1: Undated letter (fragment), ca 1867 208 Appendix 2: Obituary for Sir Julius von Haast 209 Appendix 3: Biographical register of names mentioned in the correspondence 213 Figures Figure 1: Photographs -
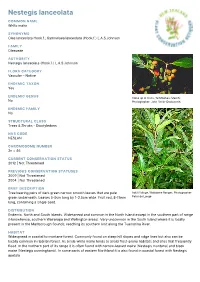
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

PLANT of the MONTH – LITSEA CALICARIS Plant of the Month for September Is Litsea Calicaris (Mangeao, Tangeao)
E-newsletter: No 106. September 2012 Deadline for next issue: Monday 15 October 2012 President’s message The New Zealand Plant Conservation Network will be 10 years old in April! It is hard to believe that the Network will have been in place for a decade, but so much has been achieved in that time. Around 2,000 people read this newsletter and our website use is huge. The 7,300 plant species pages, 23,000 plant images, 1.4 million plant distribution records and other website information/features provide a very valuable plant conservation resource that did not exist before the Network began. We will be celebrating this birthday with a conference, so book that May date in your diaries (see later in this newsletter). Also in this edition, are details of another major celebration (75 years this time!)— Auckland Botanical Society’s Diamond Jubilee Celebrations will feature a programme of lectures and a celebration dinner in late October (see Events for more information). This month, we have an article about a fantastic find on Banks Peninsula. Pittosporm obcordatum has been rediscovered after 170 years—well done Melissa. Two new features have been added to the website; an illustrated glossary and species pages of all of New Zealand’s marine algae, so check them out. We are calling for nominations for the NZPCN Plant Conservation Awards. I’m sure that you know of a person, group, council, school or nursery that you could nominate for the great work they are doing for plant conservation. Finally, look out for our AGM invitation in the next newsletter. -

Council Member Guest Editorial
E-newsletter: No 107. October 2010 Deadline for next issue: Wednesday 14 November 2012 Council member guest editorial In 2005, I remember giving a presentation to my fellow reference librarians at Christchurch City Libraries on a range of electronic resources providing information about New Zealand’s environment. The Network’s website particularly impressed me and it wasn’t long before I signed up as a member. Back then, I never would have predicted that I would have the opportunity to contribute to that impressive website, but here I am doing just that! As many of you will have read in the August issue of Trilepidea, the Network’s website is undergoing some redevelopment to improve usability. I am enjoying the challenge of helping to improve access to the amazing trove of information contained on our website. I’ve rediscovered hidden corners of the website I’d forgotten about and great new additions such as the lichens and macroalgae species pages. The amazing range of images contributed by hundreds of photographers is a powerful resource that will feature more prominently in the redeveloped site. The new site will be launched soon and I am looking forward to hearing what people think about the changes. Keep your ears and eyes open for the new website and please send your feedback to: [email protected]. There will also be some exciting changes to the Favourite Native Plant vote this year. Though some people may find this annual exercise a bit frivolous, I think it provides a good excuse to share our love for native plants with everyone. -

Researchcommons.Waikato.Ac.Nz
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Research Commons@Waikato http://researchcommons.waikato.ac.nz/ Research Commons at the University of Waikato Copyright Statement: The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). The thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. Authors control the copyright of their thesis. You will recognise the author’s right to be identified as the author of the thesis, and due acknowledgement will be made to the author where appropriate. You will obtain the author’s permission before publishing any material from the thesis. Identifying Host Species of Dactylanthus taylorii using DNA Barcoding A thesis submitted in partial fulfilment of the requirements for the degree of Masters of Science in Biological Sciences at The University of Waikato by Cassarndra Marie Parker _________ The University of Waikato 2015 Acknowledgements: This thesis wouldn't have been possible without the support of many people. Firstly, my supervisors Dr Chrissen Gemmill and Dr Avi Holzapfel - your professional expertise, advice, and patience were invaluable. From pitching the idea in 2012 to reading through drafts in the final fortnight, I've been humbled to work with such dedicated and accomplished scientists. Special mention also goes to Thomas Emmitt, David Mudge, Steven Miller, the Auckland Zoo horticulture team and Kevin. -

Araliaceae) Roderick J
Essential Oils from the Leaves of Three New Zealand Species of Pseudopanax (Araliaceae) Roderick J. Weston Industrial Research Ltd., P.O. Box 31-310, Lower Hutt, New Zealand. Fax: +64-4-9313-055. E-mail: [email protected] Z. Naturforsch. 59c, 39Ð42 (2004); received July 22, 2003 Essential oils from three of the eleven endemic New Zealand species of Pseudopanax, P. arboreus, P. discolor and P. lessonii, were found to have a fairly uniform composition which was different from that of the oils of Raukaua species that were formerly classified in the Pseudopanax genus. Oils of the three Pseudopanax species all contained significant propor- tions of viridiflorol and a closely related unidentified hydroazulene alcohol in common. In addition, the oil of P. arboreus contained bicyclogermacrene, linalool and long chain hy- drocarbons. The oil of P. discolor contained nerolidol in abundance (36.3%) together with linalool and epi-α-muurolol. The oil of P. lessonii contained a complex mixture of sesquiter- pene alcohols including epi-α-muurolol and a mixture of long chain hydrocarbons. Nerolidol and linalool provided the oil of P. discolor with a pleasant floral aroma, but the yield of oil was very low (0.01%). Key words: Pseudopanax arboreus, discolor and lessonii, Araliaceae, Essential Oil Introduction species studied in this paper, P. lessonii and P. dis- color, belong to this group and were selected be- The Araliaceae is a family of 65 genera and ap- cause their leaves, when crushed, emit a weak fra- proximately 800 species, which occur mainly in grance. The third group is characterized by its tropical regions, but some genera are found in shorter wider fleshier leaves and includes P. -

UNITED STATES DEPARTMENT (Ff AGRICULTURE
UNITED STATES DEPARTMENT (ff AGRICULTURE INVENTORY No. Washington, D. C. T Issued February, 1930 PLANT MATERIAL INTRODUCED BY THE OFFICE OF FOREIGN PLANT INTRODUCTION, BUREAU OF PLANT INDUSTRY, JULY 1 TO SEPTEMBER 30,1928 (NOS. 77261 TO 77595) CONTENTS Page Introductory statement 1 Inventory 3 Index of common and scientific names 19 INTRODUCTORY STATEMENT The most outstanding plant material contained in this inventory, No. 96, covering the period from July 1 to September 30, 1928, is the collection of 51 sugarcane varieties (Saccarum offvoinarum, Nos. 77334 to 77384) procured by E. W. Brandes in New Guinea for use in his official investigations. Part of the interest in this shipment is due to the fact that Doctor Brandes is the first agricultural explorer to use an airplane for his collecting tour. Doctor Brandes not only collected sugarcanes in person but also obtained the cooper- ation of P. H. Goldfinch, Sydney, Australia, who sent in a collection of 44 varieties (Nos. 77496 to 77539). Another lot of five varieties (Nos. 77298 to 77302) was received from Argentina. As in the previous inventory, the bulk of the plant material received in this period comes from the Southern Hemisphere. Through the activities of Mrs. Frieda Cobb Blanchard two collections of Australian plants (Nos. 77273 to 77292 and Nos. 77441 to 77447), as well as a collection from New Zealand (Nos. 77540 to 77582), were received. Through O. F. Cook there was received a collection of rubber-producing plants (Nos. 77387 to 77394) from Haiti. Five kinds of cover crops (Nos. 77293 to 77297) from Ceylon may be of value for the southern United States. -

Doctorat De L'université De Toulouse
En vue de l’obt ention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par : Université Toulouse 3 Paul Sabatier (UT3 Paul Sabatier) Discipline ou spécialité : Ecologie, Biodiversité et Evolution Présentée et soutenue par : Joeri STRIJK le : 12 / 02 / 2010 Titre : Species diversification and differentiation in the Madagascar and Indian Ocean Islands Biodiversity Hotspot JURY Jérôme CHAVE, Directeur de Recherches CNRS Toulouse Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Frédéric MEDAIL, Professeur à l'Université Paul Cezanne Aix-Marseille Christophe THEBAUD, Professeur à l'Université Paul Sabatier Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : UMR 5174 CNRS-UPS Evolution & Diversité Biologique Directeur(s) de Thèse : Christophe THEBAUD Rapporteurs : Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Contents. CONTENTS CHAPTER 1. General Introduction 2 PART I: ASTERACEAE CHAPTER 2. Multiple evolutionary radiations and phenotypic convergence in polyphyletic Indian Ocean Daisy Trees (Psiadia, Asteraceae) (in preparation for BMC Evolutionary Biology) 14 CHAPTER 3. Taxonomic rearrangements within Indian Ocean Daisy Trees (Psiadia, Asteraceae) and the resurrection of Frappieria (in preparation for Taxon) 34 PART II: MYRSINACEAE CHAPTER 4. Phylogenetics of the Mascarene endemic genus Badula relative to its Madagascan ally Oncostemum (Myrsinaceae) (accepted in Botanical Journal of the Linnean Society) 43 CHAPTER 5. Timing and tempo of evolutionary diversification in Myrsinaceae: Badula and Oncostemum in the Indian Ocean Island Biodiversity Hotspot (in preparation for BMC Evolutionary Biology) 54 PART III: MONIMIACEAE CHAPTER 6. Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long distance dispersal, but not West Gondwana (accepted in Journal of Biogeography) 72 CHAPTER 7 General Discussion 86 REFERENCES 91 i Contents. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Patterns in Ericaceae: New Phylogenetic Analyses
BS 55 479 Origins and biogeographic patterns in Ericaceae: New insights from recent phylogenetic analyses Kathleen A. Kron and James L. Luteyn Kron, KA. & Luteyn, J.L. 2005. Origins and biogeographic patterns in Ericaceae: New insights from recent phylogenetic analyses. Biol. Skr. 55: 479-500. ISSN 0366-3612. ISBN 87-7304-304-4. Ericaceae are a diverse group of woody plants that span the temperate and tropical regions of the world. Previous workers have suggested that Ericaceae originated in Gondwana, but recent phy logenetic studies do not support this idea. The theory of a Gondwanan origin for the group was based on the concentration of species richness in the Andes, southern Africa, and the southwest Pacific islands (most of which are thought to be of Gondwanan origin). The Andean diversity is comprised primarily of Vaccinieae with more than 800 species occurring in northern South America, Central America, and the Antilles. In the Cape Region of South Africa the genus Erica is highly diverse with over 600 species currently recognized. In the southwest Pacific islands, Vac cinieae and Rhodoreae are very diverse with over 290 species of Rhododendron (sect. Vireya) and approximately 500 species of Vaccinieae (I)imorphanthera, Paphia, Vaccinium). Recent phyloge netic studies have also shown that the Styphelioideae (formerly Epacridaceae) are included within Ericaceae, thus adding a fourth extremely diverse group (approximately 520 species) in areas considered Gondwanan in origin. Phylogenetic studies of the family on a global scale, how ever, have indicated that these highly diverse “Gondwanan” groups are actually derived from within Ericaceae. Both Fitch parsimony character optimization (using MacClade 4.0) and disper- sal-vicariance analysis (DIVA) indicate that Ericaceae is Laurasian in origin.