Widespread Horizontal Transfer of Mitochondrial Genes in Flowering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Survey of Roadside Alien Plants in Hawai`I Volcanoes National Park and Adjacent Residential Areas 2001–2005
Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANts IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 Linda W. Pratt1 Keali`i F. Bio2 James D. Jacobi1 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kilauea Field Station, P.O. Box 44, Hawaii National Park, HI 96718 2 Hawai‘i Cooperative Studies Unit, University of Hawai‘i at Hilo, P.O. Box 44, Hawai‘i National Park, HI 96718 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This product was prepared under Cooperative Agreement CA03WRAG0036 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-032 SURVEY OF ROADSIDE ALIEN PLANTS IN HAWAI`I VOLCANOES NATIONAL PARK AND ADJACENT RESIDENTIAL AREAS 2001–2005 1 2 1 LINDA W. PRATT , KEALI`I F. BIO , AND JAMES D. JACOBI 1 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Kīlauea Field Station, P.O. Box 44, Hawai`i Volcanoes National Park, HI 96718 2 Hawaii Cooperative Studies Unit, University of Hawai`i at Hilo, Hilo, HI 96720 Hawai`i Cooperative Studies Unit University of Hawai`i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 September 2012 This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices ( http://pubs.usgs.gov/circ/1367/ ). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. -

The Developmental and Genetic Bases of Apetaly in Bocconia Frutescens
Arango‑Ocampo et al. EvoDevo (2016) 7:16 DOI 10.1186/s13227-016-0054-6 EvoDevo RESEARCH Open Access The developmental and genetic bases of apetaly in Bocconia frutescens (Chelidonieae: Papaveraceae) Cristina Arango‑Ocampo1, Favio González2, Juan Fernando Alzate3 and Natalia Pabón‑Mora1* Abstract Background: Bocconia and Macleaya are the only genera of the poppy family (Papaveraceae) lacking petals; how‑ ever, the developmental and genetic processes underlying such evolutionary shift have not yet been studied. Results: We studied floral development in two species of petal-less poppies Bocconia frutescens and Macleaya cordata as well as in the closely related petal-bearing Stylophorum diphyllum. We generated a floral transcriptome of B. frutescens to identify MADS-box ABCE floral organ identity genes expressed during early floral development. We performed phylogenetic analyses of these genes across Ranunculales as well as RT-PCR and qRT-PCR to assess loci- specific expression patterns. We found that petal-to-stamen homeosis in petal-less poppies occurs through distinct developmental pathways. Transcriptomic analyses of B. frutescens floral buds showed that homologs of all MADS-box genes are expressed except for the APETALA3-3 ortholog. Species-specific duplications of other ABCE genes inB. frute- scens have resulted in functional copies with expanded expression patterns than those predicted by the model. Conclusions: Petal loss in B. frutescens is likely associated with the lack of expression of AP3-3 and an expanded expression of AGAMOUS. The genetic basis of petal identity is conserved in Ranunculaceae and Papaveraceae although they have different number of AP3 paralogs and exhibit dissimilar floral groundplans. -

HAWAII and SOUTH PACIFIC ISLANDS REGION - 2016 NWPL FINAL RATINGS U.S
HAWAII and SOUTH PACIFIC ISLANDS REGION - 2016 NWPL FINAL RATINGS U.S. ARMY CORPS OF ENGINEERS, COLD REGIONS RESEARCH AND ENGINEERING LABORATORY (CRREL) - 2013 Ratings Lichvar, R.W. 2016. The National Wetland Plant List: 2016 wetland ratings. User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps region. Scientific Name Common Name Hawaii Status South Pacific Agrostis canina FACU Velvet Bent Islands Status Agrostis capillaris UPL Colonial Bent Abelmoschus moschatus FAC Musk Okra Agrostis exarata FACW Spiked Bent Abildgaardia ovata FACW Flat-Spike Sedge Agrostis hyemalis FAC Winter Bent Abrus precatorius FAC UPL Rosary-Pea Agrostis sandwicensis FACU Hawaii Bent Abutilon auritum FACU Asian Agrostis stolonifera FACU Spreading Bent Indian-Mallow Ailanthus altissima FACU Tree-of-Heaven Abutilon indicum FAC FACU Monkeybush Aira caryophyllea FACU Common Acacia confusa FACU Small Philippine Silver-Hair Grass Wattle Albizia lebbeck FACU Woman's-Tongue Acaena exigua OBL Liliwai Aleurites moluccanus FACU Indian-Walnut Acalypha amentacea FACU Alocasia cucullata FACU Chinese Taro Match-Me-If-You-Can Alocasia macrorrhizos FAC Giant Taro Acalypha poiretii UPL Poiret's Alpinia purpurata FACU Red-Ginger Copperleaf Alpinia zerumbet FACU Shellplant Acanthocereus tetragonus UPL Triangle Cactus Alternanthera ficoidea FACU Sanguinaria Achillea millefolium UPL Common Yarrow Alternanthera sessilis FAC FACW Sessile Joyweed Achyranthes -

9:00 Am PLACE
CARTY S. CHANG INTERIM CHAIRPERSON DAVID Y. IGE BOARD OF LAND AND NATURAL RESOURCES GOVERNOR OF HAWAII COMMISSION ON WATER RESOURCE MANAGEMENT KEKOA KALUHIWA FIRST DEPUTY W. ROY HARDY ACTING DEPUTY DIRECTOR – WATER AQUATIC RESOURCES BOATING AND OCEAN RECREATION BUREAU OF CONVEYANCES COMMISSION ON WATER RESOURCE MANAGEMENT STATE OF HAWAII CONSERVATION AND COASTAL LANDS CONSERVATION AND RESOURCES ENFORCEMENT DEPARTMENT OF LAND AND NATURAL RESOURCES ENGINEERING FORESTRY AND WILDLIFE HISTORIC PRESERVATION POST OFFICE BOX 621 KAHOOLAWE ISLAND RESERVE COMMISSION LAND HONOLULU, HAWAII 96809 STATE PARKS NATURAL AREA RESERVES SYSTEM COMMISSION MEETING DATE: April 27, 2015 TIME: 9:00 a.m. PLACE: Department of Land and Natural Resources Boardroom, Kalanimoku Building, 1151 Punchbowl Street, Room 132, Honolulu. AGENDA ITEM 1. Call to order, introductions, move-ups. ITEM 2. Approval of the Minutes of the June 9, 2014 N atural Area Reserves System Commission Meeting. ITEM 3. Natural Area Partnership Program (NAPP). ITEM 3.a. Recommendation to the Board of Land and Natural Resources approval for authorization of funding for The Nature Conservancy of Hawaii for $663,600 during FY 16-21 for continued enrollment in the natural area partnership program and acceptance and approval of the Kapunakea Preserve Long Range Management Plan, TMK 4-4-7:01, 4-4-7:03, Lahaina, Maui. ITEM 3.b. Recommendation to the Board of Land and Natural Resources approval for authorization of funding for The Nature Conservancy of Hawaii for $470,802 during FY 16-21 for continued enrollment in the natural area partnership program and acceptance and approval of the Pelekunu Long Range Management Plan, TMK 5-4- 3:32, 5-9-6:11, Molokai. -

Field Instructions for the Periodic Inventory of The
FIELD INSTRUCTIONS FOR THE PERIODIC INVENTORY OF THE COMMONWEALTH OF THE NORTHERN MARIANA ISLANDS 2015 FOREST INVENTORY AND ANALYSIS RESOURCE MONITORING AND ASSESSMENT PROGRAM PACIFIC NORTHWEST RESEARCH STATION USDA FOREST SERVICE THIS MANUAL IS BASED ON: FOREST INVENTORY AND ANALYSIS NATIONAL CORE FIELD GUIDE VOLUME I: FIELD DATA COLLECTION PROCEDURES VERSION 6.1 Cover image by Gretchen Bracher pg.I Table of Contents CHAPTER 1 INTRODUCTION . 15 SECTION 1.1 ORGANIZATION OF THIS MANUAL. 15 SECTION 1.2 THE INVENTORY. 16 SECTION 1.3 PRODUCTS . 16 SECTION 1.4 UNITS OF MEASURE . 16 SECTION 1.5 PLOT DESIGN GENERAL DESCRIPTION . 16 SUBSECTION 1.5.1 PLOT LAYOUT . .17 SUBSECTION 1.5.2 DATA ARE COLLECTED ON PLOTS AT THE FOLLOWING LEVELS .17 SECTION 1.6 QUALITY ASSURANCE/QUALITY CONTROL . 18 SUBSECTION 1.6.1 GENERAL DESCRIPTION . .18 SECTION 1.7 SAFETY . 18 SUBSECTION 1.7.1 SAFETY IN THE WOODS. .18 SUBSECTION 1.7.2 SAFETY ON THE ROAD . .19 SUBSECTION 1.7.3 WHAT TO DO IF INJURED. .19 CHAPTER 2 LOCATING THE PLOT . 21 SECTION 2.1 LOCATING AN ESTABLISHED PLOT . 21 SUBSECTION 2.1.1 NAVIGATING WITH PHOTOGRAPHY. .21 SUBSECTION 2.1.2 NAVIGATING WITH GPS . .21 SUBSECTION 2.1.3 NAVIGATING WITH REFERENCE POINT (RP) DATA . .22 SUBSECTION 2.1.4 REVERSE REFERENCE POINT (RP) METHOD . .22 SECTION 2.2 ESTABLISHED PLOT ISSUES . 22 SUBSECTION 2.2.1 DIFFICULTY FINDING ESTABLISHED PLOTS. 22 SUBSECTION 2.2.2 INCORRECTLY INSTALLED PLOT . .23 SUBSECTION 2.2.3 INCORRECTLY INSTALLED SUBPLOT OR MICROPLOT. .23 SUBSECTION 2.2.4 PC STAKE OR SUBPLOT/MICROPLOT PIN MISSING OR MOVED . -
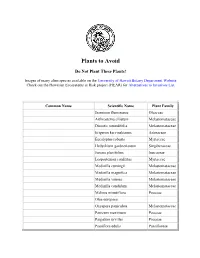
Plants to Avoid
Plants to Avoid Do ot Plant These Plants! Images of many alien species available on the University of Hawaii Botany Department Website Check out the Hawaiian Ecosystems at Risk project (HEAR) for Alternatives to Invasives List Common ame Scientific ame Plant Family Jasminim fluminense Oleaceae Arthrostema ciliatum Melastomataceae Dissotis rotundifolia Melastomataceae Erigeron karvinskianus Asteraceae Eucalyptus robusta Myrtaceae Hedychium gardnerianum Singiberaceae Juncus planifolius Juncaceae Lospostemon confertus Myrtaceae Medinilla cumingii Melastomataceae Medinilla magnifica Melastomataceae Medinilla venosa Melastomataceae Medinilla candidum Melastomataceae Melinis minutiflora Poaceae Olea europaea Oxyspora paniculata Melastomataceae Panicum maximum Poaceae Paspalum urvillei Poaceae Passiflora edulis Passifloreae Phormium tenax Agavaceae Pinus taeda Pinaceae Prosopis pallida Favaceae Pterolepis glomerata Melastomataceae Rhodomyrtus tomentosa Myrtaceae Schefflera actinophylla Araliaceae Syzygium jambos Myrtaceae Australian blackwood Acacia melanoxylon Mimosaceae Australian tree fern Cyathea cooperi Cyatheaceae Australian tree fern Sphaeropteris cooperi Cyatheaceae Beggar's tick, Spanish needle Bidens pilosa Asteraceae California grass Brachiaria mutica Poaceae Chinese banyan, Malayan banyan Ficus mirocarpa Moraceae Chinese violet Asystasia gangetica Acanthaceae Christmasberry, Brazilian pepper Schinus terebinthifolius Anacardiaceae Formosan koa Acacia confusa Mimosaceae German ivy Senecio mikanioides Asteraceae Japanese honeysuckle -

Implementing Early Detection in Hawai”I, Year One
Implementing Early Detection in Hawai”i, Year One Final Report prepared for: Hawaii Invasive Species Council Research and Technology Program Department of Land and Natural Resources, Division of Forestry and Wildlife Prepared by: Clyde T. Imada, Danielle Frohlich, Alex Lau, and Ryan Smith December 2007 Hawaii Biological Survey Report 2007-016 Implementing Early Detection in Hawai”i, Year One TABLE OF CONTENTS Table of Contents ........................................................................................................................................... i Executive Summary ....................................................................................................................................... 1 I. Introduction ................................................................................................................................................ 2 II. Detection Plan Model ............................................................................................................................... 4 IIa. Building a Target Species List .................................................................................................. 4 IIb. High-risk Sites and Survey Methodology ................................................................................. 6 IIc. Prioritizing for Control ............................................................................................................. 8 IId. Targeted Roadside Surveys ................................................................................................... -

Recovery Plan for the Maui Plant Cluster (Hawaii)
Recovery Plan for the Maui Plant Cluster (Hawaii) US Department of the Interior Fish and Wildlife Service Portland, Oregon July 1997 RECOVERY PLAN FOR THE MAUI PLANT CLUSTER Published By U.S. Fish and Wildlife Service Portland, Oregon ~Lao~J. ~ Approved: Regi al Director, U.S. F ildlife Service Date: DISCLAIMER Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance ofrecovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Costs indicated for task implementation and/or time for achievement ofrecovery are only estimates and subject to change. Recovery plans do not necessarily represent the views, official positions nor approval ofany individuals or agencies involved in the plan formulation, other than the U.S. Fish and Wildlife Service. They represent the official position ofthe U.S. Fish and Wildlife Service only after they have been signed by the Regional Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion ofrecovery tasks. LITERATURE CITATION: U.S. Fish and Wildlife Service. 1997. Recovery Plan for the Maui Plant Cluster. U.S. Fish and Wildlife Service, Portland, OR. 130 pp. + appendices ADDITIONAL COPIES MAY BE PURCHASED FROM: Fish and Wildlife Reference Service 5430 Grosvenor Lane, Suite 110 Bethesda, Maryland 20814 telephone: 301/492-6403 or 1-800-582-3421 fax: 301/564-4059 e-mail: fwrs~mail.fws.gov Fees for plans vary depending on the number of pages. -

WUCOLS List S Abelia Chinensis Chinese Abelia M ? ? M / / Copyright © UC Regents, Davis Campus
Ba Bu G Gc P Pm S Su T V N Botanical Name Common Name 1 2 3 4 5 6 Symbol Vegetation Used in Type WUCOLS List S Abelia chinensis Chinese abelia M ? ? M / / Copyright © UC Regents, Davis campus. All rights reserved. bamboo Ba S Abelia floribunda Mexican abelia M ? M M / / S Abelia mosanensis 'Fragrant Abelia' fragrant abelia ? ? ? ? ? ? bulb Bu S Abelia parvifolia (A. longituba) Schuman abelia ? ? ? M ? ? grass G groundcover GC Gc S Abelia x grandiflora and cvs. glossy abelia M M M M M / perennial* P S Abeliophyllum distichum forsythia M M ? ? ? ? palm and cycad Pm S Abelmoschus manihot (Hibiscus manihot) sunset muskmallow ? ? ? L ? ? T Abies pinsapo Spanish fir L L L / / / shrub S succulent Su T N Abies spp. (CA native and non-native) fir M M M M / / P N Abronia latifolia yellow sand verbena VL VL VL / ? ? tree T P N Abronia maritima sand verbena VL VL VL / ? ? vine V California N native S N Abutilon palmeri Indian mallow L L L L M M S Abutilon pictum thompsonii variegated Chinese lantern M H M M ? ? Sunset WUCOLS CIMIS ET Representative Number climate 0 Region zones** Cities zones* S Abutilon vitifolium flowering maple M M M / ? ? Healdsburg, Napa, North- San Jose, Salinas, Central 14, 15, 16, 17 1, 2, 3, 4, 6, 8 San Francisco, Coastal San Luis Obispo S Abutilon x hybridum & cvs. flowering maple M H M M / / 1 Auburn, Central Bakersfield, Chico, 8, 9, 14 12, 14, 15, 16 Valley Fresno, Modesto, Sacramento S T Acacia abyssinica Abyssinian acacia / ? / ? / L 2 Irvine, Los South Angeles, Santa 22, 23, 24 1, 2, 4, 6 Coastal Barbara, Ventura, -
Session 5: Prospects for Weed Biological Control in Pacific Islands 206 Session 5 Prospects for Weed Biological Control in Pacific Islands
Session 5: Prospects for Weed Biological Control in Pacific Islands 206 Session 5 Prospects for Weed Biological Control in Pacific Islands Weeds of Hawaii’s Lands Devoted to Watershed Protection and Biodiversity Conservation: Role of Biological Control as the Missing Piece in an Integrated Pest Management Strategy A. C. Medeiros and L. L. Loope U.S. Geological Survey, Pacific Island Ecosystems Research Center, Makawao, HI 96768 USA email: [email protected]; [email protected] Summary Despite Hawaii’s reputation as an extinction icon, significant biological resources remain, especially in watersheds, natural areas, and specialized edaphic sites (e.g., lava dry forest, coastal). While direct habitat destruction by humans continues, human-facilitated biological invaders are currently the primary agents of continuing degradation. The ability of invasive plants to have prolific seed production, efficient dispersal systems, and to become established in dense vegetation, complicated by Hawaii’s rugged topography, appears to render mechanical and chemical control as mere holding actions. Costly, ‘environmentally unfriendly’, and often ineffective, strategies using chemical and mechanical control on a large scale, despite the most valiant of efforts, can be viewed simply as attempts to buy time. Without increased levels of safely tested biological control, the seemingly inevitable result is the landscape level transformation of native forests, with potentially catastrophic consequences to cultural, biological, water, and economic resources. Increased levels of effective biological control for certain intractable invasive species appear to comprise a conspicuous ‘missing piece’ in our efforts to protect Hawaiian watersheds and other conservation lands. Evolution of Hawaiian Biota are all apparent drivers of the development of Hawaii’s renowned biota and perhaps its relative vulnerability to invasions (Loope, 2011). -

WRA Species Report
Family: Papaveraceae Taxon: Bocconia frutescens Synonym: Bocconia integrifolia Bonpl. Common Name: Plume poppy Bocconia frutescens var. cernua Moc. & Sessé Tree poppy Bocconia glauca Salisb. bocconia Bocconia pearcei Hutch. parrotweed Bocconia quercifolia Moench Bocconia sinuatifolia Stokes Bocconia subtomentosa L'Hér. ex Stahl Questionaire : current 20090513 Assessor: Chuck Chimera Designation: H(Hawai'i) Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 18 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 n 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see y Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see y Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier -

Survey for Natural Enemies of Bocconia Frutescens in Costa Rica
252 Session 5 Prospects for Weed Biological Control in Pacific Islands Survey for Natural Enemies of Bocconia frutescens in Costa Rica K. Nishida1 and M. T. Johnson2 1Universidad de Costa Rica, Escuela de Biología, San José, Costa Rica [email protected] 2USDA Forest Service, Pacific Southwest Research Station, Institute of Pacific Islands Forestry, Volcano, HI, USA Abstract Bocconia (Bocconia frutescens L.) (Papaveraceae) are small trees up to 6 m tall native to the Neotropics. Bocconia has established populations on the islands of Maui and Hawaii, spreading from dense groves in disturbed sites to invade native dryland and mesic forests, where it is considered a threat to rare endemic organisms. Bocconia is recognized as a noxious weed by the State of Hawaii, making it a target for eradication on islands where it is not already established. Mechanical and chemical controls have been pursued in some areas, but are prohibitively expensive over its present wide distribution. Bocconia is found in a variety of habitats in biodiversity-protected land of Costa Rica, typically colonizing disturbed areas such as tree falls and landslides but without displaying invasiveness. Plants in this native range were explored for insects and other natural enemies between December 2008 and September 2009. On monthly survey trips habitat and plant conditions were noted, and its natural enemies were photographed and collected for rearing and identification. A total of 38 species of natural enemies have been documented (35 insects, one mite, one phytoplasma and one fungus). Thus far only three potential control agents are recognized: a gregarious leafminer (Liriomyza sp.) (Agromyzidae), a treehopper (Ennya pacifica Fairmaire) (Membracidae) which deposits egg masses in leaf veins and inflorescence stems, and an unidentified leaf tier moth (Tortricidae) whose larvae damage young leaves.