Antibiotic Discovery: History, Methods and Perspectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Antibiotic Consumption for Human Use in Sierra Leone (2017–2019): a Cross-Sectional Study
Tropical Medicine and Infectious Disease Article National Antibiotic Consumption for Human Use in Sierra Leone (2017–2019): A Cross-Sectional Study Joseph Sam Kanu 1,2,* , Mohammed Khogali 3, Katrina Hann 4 , Wenjing Tao 5, Shuwary Barlatt 6,7, James Komeh 6, Joy Johnson 6, Mohamed Sesay 6, Mohamed Alex Vandi 8, Hannock Tweya 9, Collins Timire 10, Onome Thomas Abiri 6,11 , Fawzi Thomas 6, Ahmed Sankoh-Hughes 12, Bailah Molleh 4, Anna Maruta 13 and Anthony D. Harries 10,14 1 National Disease Surveillance Programme, Sierra Leone National Public Health Emergency Operations Centre, Ministry of Health and Sanitation, Cockerill, Wilkinson Road, Freetown, Sierra Leone 2 Department of Community Health, Faculty of Clinical Sciences, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone 3 Special Programme for Research and Training in Tropical Diseases (TDR), World Health Organization, 1211 Geneva, Switzerland; [email protected] 4 Sustainable Health Systems, Freetown, Sierra Leone; [email protected] (K.H.); [email protected] (B.M.) 5 Unit for Antibiotics and Infection Control, Public Health Agency of Sweden, Folkhalsomyndigheten, SE-171 82 Stockholm, Sweden; [email protected] 6 Pharmacy Board of Sierra Leone, Central Medical Stores, New England Ville, Freetown, Sierra Leone; [email protected] (S.B.); [email protected] (J.K.); [email protected] (J.J.); [email protected] (M.S.); [email protected] (O.T.A.); [email protected] (F.T.) Citation: Kanu, J.S.; Khogali, M.; 7 Department of Pharmaceutics and Clinical Pharmacy & Therapeutics, Faculty of Pharmaceutical Sciences, Hann, K.; Tao, W.; Barlatt, S.; Komeh, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown 0000, Sierra Leone 8 J.; Johnson, J.; Sesay, M.; Vandi, M.A.; Directorate of Health Security & Emergencies, Ministry of Health and Sanitation, Sierra Leone National Tweya, H.; et al. -

Novel Antimicrobial Agents Inhibiting Lipid II Incorporation Into Peptidoglycan Essay MBB
27 -7-2019 Novel antimicrobial agents inhibiting lipid II incorporation into peptidoglycan Essay MBB Mark Nijland S3265978 Supervisor: Prof. Dr. Dirk-Jan Scheffers Molecular Microbiology University of Groningen Content Abstract..............................................................................................................................................2 1.0 Peptidoglycan biosynthesis of bacteria ........................................................................................3 2.0 Novel antimicrobial agents ...........................................................................................................4 2.1 Teixobactin ...............................................................................................................................4 2.2 tridecaptin A1............................................................................................................................7 2.3 Malacidins ................................................................................................................................8 2.4 Humimycins ..............................................................................................................................9 2.5 LysM ........................................................................................................................................ 10 3.0 Concluding remarks .................................................................................................................... 11 4.0 references ................................................................................................................................. -

Brilacidin First-In-Class Defensin-Mimetic Drug Candidate
Brilacidin First-in-Class Defensin-Mimetic Drug Candidate Mechanism of Action, Pre/Clinical Data and Academic Literature Supporting the Development of Brilacidin as a Potential Novel Coronavirus (COVID-19) Treatment April 20, 2020 Page # I. Brilacidin: Background Information 2 II. Brilacidin: Two Primary Mechanisms of Action 3 Membrane Disruption 4 Immunomodulatory 7 III. Brilacidin: Several Complementary Ways of Targeting COVID-19 10 Antiviral (anti-SARS-CoV-2 activity) 11 Immuno/Anti-Inflammatory 13 Antimicrobial 16 IV. Brilacidin: COVID-19 Clinical Development Pathways 18 Drug 18 Vaccine 20 Next Steps 24 V. Brilacidin: Phase 2 Clinical Trial Data in Other Indications 25 VI. AMPs/Defensins (Mimetics): Antiviral Properties 30 VII. AMPs/Defensins (Mimetics): Anti-Coronavirus Potential 33 VIII. The Broader Context: Characteristics of the COVID-19 Pandemic 36 Innovation Pharmaceuticals 301 Edgewater Place, Ste 100 Wakefield, MA 01880 978.921.4125 [email protected] Innovation Pharmaceuticals: Mechanism of Action, Pre/Clinical Data and Academic Literature Supporting the Development of Brilacidin as a Potential Novel Coronavirus (COVID-19) Treatment (April 20, 2020) Page 1 of 45 I. Brilacidin: Background Information Brilacidin (PMX-30063) is Innovation Pharmaceutical’s lead Host Defense Protein (HDP)/Defensin-Mimetic drug candidate targeting SARS-CoV-2, the virus responsible for COVID-19. Laboratory testing conducted at a U.S.-based Regional Biocontainment Laboratory (RBL) supports Brilacidin’s antiviral activity in directly inhibiting SARS-CoV-2 in cell-based assays. Additional pre-clinical and clinical data support Brilacidin’s therapeutic potential to inhibit the production of IL-6, IL-1, TNF- and other pro-inflammatory cytokines and chemokines (e.g., MCP-1), identified as central drivers in the worsening prognoses of COVID-19 patients. -

100 Most Important Chemical Compounds
69. Penicillin CHEMICAL NAME = 2S,5R,6R)-3,3-dimeth- yl-7-oxo-6-[(phenylacetyl)amino]-4-thia-1- azabicyclo[3.2.0]heptane-2-carboxylic acid CAS NUMBER = 61–33–6 = MOLECULAR FORMULA C6H18N2O4S MOLAR MASS = 334.4 g/mol COMPOSITION = C(57.5%) H(5.4%) N(8.4%) O(19.1%) S(9.6%) MELTING POINT = 209–212°C (for Penicillin G sodium) BOILING POINT = decomposes DENSITY = 1.4 g/cm3 Penicillin was the fi rst natural antibiotic used to treat bacterial infections and continues to be one of the most important antibiotics. Th e name comes from the fungus genus Penicillium from which it was isolated. Penicillus is Latin for “brush” and refers to the brushlike appear- ance of fi lamentous Penicillium species. Species of this genus are quite common and appear as the bluish-green mold that appears on aged bread, fruit, and cheese. Th e term penicillin is a generic term that refers to a number of antibiotic compounds with the same basic structure. Th erefore it is more appropriate to speak of penicillins than of penicillin. Th e general penicil- lin structure consists of a β-lactam ring and thiazolidine ring fused together with a peptide bonded to a variable R group (Figure 69.1). Penicillin belongs to a group of compounds called β-lactam antibiotics. Diff erent forms of penicillin depend on what R group is bonded to this basic structure. Penicillin aff ects the cell walls of bacteria. Th e β-lactam rings in penicillins open in the presence of bacteria enzymes that are essential for cell wall formation. -

Infant Antibiotic Exposure Search EMBASE 1. Exp Antibiotic Agent/ 2
Infant Antibiotic Exposure Search EMBASE 1. exp antibiotic agent/ 2. (Acedapsone or Alamethicin or Amdinocillin or Amdinocillin Pivoxil or Amikacin or Aminosalicylic Acid or Amoxicillin or Amoxicillin-Potassium Clavulanate Combination or Amphotericin B or Ampicillin or Anisomycin or Antimycin A or Arsphenamine or Aurodox or Azithromycin or Azlocillin or Aztreonam or Bacitracin or Bacteriocins or Bambermycins or beta-Lactams or Bongkrekic Acid or Brefeldin A or Butirosin Sulfate or Calcimycin or Candicidin or Capreomycin or Carbenicillin or Carfecillin or Cefaclor or Cefadroxil or Cefamandole or Cefatrizine or Cefazolin or Cefixime or Cefmenoxime or Cefmetazole or Cefonicid or Cefoperazone or Cefotaxime or Cefotetan or Cefotiam or Cefoxitin or Cefsulodin or Ceftazidime or Ceftizoxime or Ceftriaxone or Cefuroxime or Cephacetrile or Cephalexin or Cephaloglycin or Cephaloridine or Cephalosporins or Cephalothin or Cephamycins or Cephapirin or Cephradine or Chloramphenicol or Chlortetracycline or Ciprofloxacin or Citrinin or Clarithromycin or Clavulanic Acid or Clavulanic Acids or clindamycin or Clofazimine or Cloxacillin or Colistin or Cyclacillin or Cycloserine or Dactinomycin or Dapsone or Daptomycin or Demeclocycline or Diarylquinolines or Dibekacin or Dicloxacillin or Dihydrostreptomycin Sulfate or Diketopiperazines or Distamycins or Doxycycline or Echinomycin or Edeine or Enoxacin or Enviomycin or Erythromycin or Erythromycin Estolate or Erythromycin Ethylsuccinate or Ethambutol or Ethionamide or Filipin or Floxacillin or Fluoroquinolones -
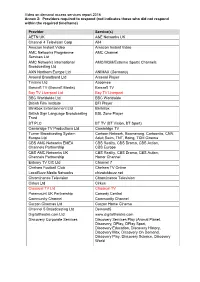
Annex 2: Providers Required to Respond (Red Indicates Those Who Did Not Respond Within the Required Timeframe)
Video on demand access services report 2016 Annex 2: Providers required to respond (red indicates those who did not respond within the required timeframe) Provider Service(s) AETN UK A&E Networks UK Channel 4 Television Corp All4 Amazon Instant Video Amazon Instant Video AMC Networks Programme AMC Channel Services Ltd AMC Networks International AMC/MGM/Extreme Sports Channels Broadcasting Ltd AXN Northern Europe Ltd ANIMAX (Germany) Arsenal Broadband Ltd Arsenal Player Tinizine Ltd Azoomee Barcroft TV (Barcroft Media) Barcroft TV Bay TV Liverpool Ltd Bay TV Liverpool BBC Worldwide Ltd BBC Worldwide British Film Institute BFI Player Blinkbox Entertainment Ltd BlinkBox British Sign Language Broadcasting BSL Zone Player Trust BT PLC BT TV (BT Vision, BT Sport) Cambridge TV Productions Ltd Cambridge TV Turner Broadcasting System Cartoon Network, Boomerang, Cartoonito, CNN, Europe Ltd Adult Swim, TNT, Boing, TCM Cinema CBS AMC Networks EMEA CBS Reality, CBS Drama, CBS Action, Channels Partnership CBS Europe CBS AMC Networks UK CBS Reality, CBS Drama, CBS Action, Channels Partnership Horror Channel Estuary TV CIC Ltd Channel 7 Chelsea Football Club Chelsea TV Online LocalBuzz Media Networks chizwickbuzz.net Chrominance Television Chrominance Television Cirkus Ltd Cirkus Classical TV Ltd Classical TV Paramount UK Partnership Comedy Central Community Channel Community Channel Curzon Cinemas Ltd Curzon Home Cinema Channel 5 Broadcasting Ltd Demand5 Digitaltheatre.com Ltd www.digitaltheatre.com Discovery Corporate Services Discovery Services Play -

Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020
First independent framework for assessing pharmaceutical company action Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020 ACKNOWLEDGEMENTS The Access to Medicine Foundation would like to thank the following people and organisations for their contributions to this report.1 FUNDERS The Antimicrobial Resistance Benchmark research programme is made possible with financial support from UK AID and the Dutch Ministry of Health, Welfare and Sport. Expert Review Committee Research Team Reviewers Hans Hogerzeil - Chair Gabrielle Breugelmans Christine Årdal Gregory Frank Fatema Rafiqi Karen Gallant Nina Grundmann Adrián Alonso Ruiz Hans Hogerzeil Magdalena Kettis Ruth Baron Hitesh Hurkchand Joakim Larsson Dulce Calçada Joakim Larsson Marc Mendelson Moska Hellamand Marc Mendelson Margareth Ndomondo-Sigonda Kevin Outterson Katarina Nedog Sarah Paulin (Observer) Editorial Team Andrew Singer Anna Massey Deirdre Cogan ACCESS TO MEDICINE FOUNDATION Rachel Jones The Access to Medicine Foundation is an independent Emma Ross non-profit organisation based in the Netherlands. It aims to advance access to medicine in low- and middle-income Additional contributors countries by stimulating and guiding the pharmaceutical Thomas Collin-Lefebvre industry to play a greater role in improving access to Alex Kong medicine. Nestor Papanikolaou Address Contact Naritaweg 227-A For more information about this publication, please contact 1043 CB, Amsterdam Jayasree K. Iyer, Executive Director The Netherlands [email protected] +31 (0) 20 215 35 35 www.amrbenchmark.org 1 This acknowledgement is not intended to imply that the individuals and institutions referred to above endorse About the cover: Young woman from the Antimicrobial Resistance Benchmark methodology, Brazil, where 40%-60% of infections are analyses or results. -

Configuration Manager User Guide © 2019 Quest Software Inc
Quest® Enterprise Reporter 3.2.1 Configuration Manager User Guide © 2019 Quest Software Inc. ALL RIGHTS RESERVED. This guide contains proprietary information protected by copyright. The software described in this guide is furnished under a software license or nondisclosure agreement. This software may be used or copied only in accordance with the terms of the applicable agreement. No part of this guide may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying and recording for any purpose other than the purchaser’s personal use without the written permission of Quest Software Inc. The information in this document is provided in connection with Quest Software products. No license, express or implied, by estoppel or otherwise, to any intellectual property right is granted by this document or in connection with the sale of Quest Software products. EXCEPT AS SET FORTH IN THE TERMS AND CONDITIONS AS SPECIFIED IN THE LICENSE AGREEMENT FOR THIS PRODUCT, QUEST SOFTWARE ASSUMES NO LIABILITY WHATSOEVER AND DISCLAIMS ANY EXPRESS, IMPLIED OR STATUTORY WARRANTY RELATING TO ITS PRODUCTS INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT. IN NO EVENT SHALL QUEST SOFTWARE BE LIABLE FOR ANY DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE, SPECIAL OR INCIDENTAL DAMAGES (INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF PROFITS, BUSINESS INTERRUPTION OR LOSS OF INFORMATION) ARISING OUT OF THE USE OR INABILITY TO USE THIS DOCUMENT, EVEN IF QUEST SOFTWARE HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. Quest Software makes no representations or warranties with respect to the accuracy or completeness of the contents of this document and reserves the right to make changes to specifications and product descriptions at any time without notice. -

AMEG Categorisation of Antibiotics
12 December 2019 EMA/CVMP/CHMP/682198/2017 Committee for Medicinal Products for Veterinary use (CVMP) Committee for Medicinal Products for Human Use (CHMP) Categorisation of antibiotics in the European Union Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 29 October 2018 Adopted by the CVMP for release for consultation 24 January 2019 Adopted by the CHMP for release for consultation 31 January 2019 Start of public consultation 5 February 2019 End of consultation (deadline for comments) 30 April 2019 Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 19 November 2019 Adopted by the CVMP 5 December 2019 Adopted by the CHMP 12 December 2019 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Categorisation of antibiotics in the European Union Table of Contents 1. Summary assessment and recommendations .......................................... 3 2. Introduction ............................................................................................ 7 2.1. Background ........................................................................................................ -

Conjunctivitis Or Worse?
Red Eye in Dogs and CatS: Conjunctivitis or Worse? Tracy Revoir, DVM Senior Manager of Veterinary Support, Dechra Veterinary Products It should come as no surprise that conjunctivitis is Common Causes of Conjunctivitis the most common ophthalmic disorder in dogs and cats. But because the clinical signs of conjunctivitis If you do confirm conjunctivitis, the next step is can mimic those of more serious ophthalmic identifying the cause. If both eyes are affected and diseases (glaucoma and uveitis), it’s important to abnormal clinical signs are apparent in other body confirm your diagnosis. systems, think underlying systemic disease. If only one eye is affected, rule out infection, tear film What are important clues to the severity of the deficiencies, an irritant, anatomical abnormality, condition? With conjunctivitis, the inflammation or deeper ocular disease. should be limited to the conjunctiva. Hyperemic conjunctival vessels are superficial, branching, In dogs, conjunctivitis can result from anatomical and bright red. They are movable over the deeper disorders, irritants, infection (usually bacterial), or episcleral vessels and can be blanched with topical atopy. Most bacterial infections are secondary dilute phenylephrine. With glaucoma and uveitis, the conditions, most often to allergies. In cats, herpes- episcleral vessels are engorged; they are dark red, virus and Chlamydophila felis are the most common deep, straight, and immobile and do not blanch with causes of conjunctivitis. Atopy can also be topical dilute phenylephrine. With conjunctivitis, an issue in cats. the Schirmer tear test and intraocular pressures are normal. And the cornea should be clear and no aqueous flare should be present. The pupil and Addressing the Problem pupillary responses are normal and intraocular structures should be visible. -
Channel Guide August 2018
CHANNEL GUIDE AUGUST 2018 KEY HOW TO FIND WHICH CHANNELS YOU HAVE 1 PLAYER PREMIUM CHANNELS 1. Match your ENTERTAINMENT package 1 2 3 4 5 6 2 MORE to the column 100 Virgin Media Previews 3 M+ 101 BBC One If there’s a tick 4 MIX 2. 102 BBC Two in your column, 103 ITV 5 FUN you get that 104 Channel 4 6 FULL HOUSE channel ENTERTAINMENT SPORT 1 2 3 4 5 6 1 2 3 4 5 6 100 Virgin Media Previews 501 Sky Sports Main Event 101 BBC One HD 102 BBC Two 502 Sky Sports Premier 103 ITV League HD 104 Channel 4 503 Sky Sports Football HD 105 Channel 5 504 Sky Sports Cricket HD 106 E4 505 Sky Sports Golf HD 107 BBC Four 506 Sky Sports F1® HD 108 BBC One HD 507 Sky Sports Action HD 109 Sky One HD 508 Sky Sports Arena HD 110 Sky One 509 Sky Sports News HD 111 Sky Living HD 510 Sky Sports Mix HD 112 Sky Living 511 Sky Sports Main Event 113 ITV HD 512 Sky Sports Premier 114 ITV +1 League 115 ITV2 513 Sky Sports Football 116 ITV2 +1 514 Sky Sports Cricket 117 ITV3 515 Sky Sports Golf 118 ITV4 516 Sky Sports F1® 119 ITVBe 517 Sky Sports Action 120 ITVBe +1 518 Sky Sports Arena 121 Sky Two 519 Sky Sports News 122 Sky Arts 520 Sky Sports Mix 123 Pick 521 Eurosport 1 HD 132 Comedy Central 522 Eurosport 2 HD 133 Comedy Central +1 523 Eurosport 1 134 MTV 524 Eurosport 2 135 SYFY 526 MUTV 136 SYFY +1 527 BT Sport 1 HD 137 Universal TV 528 BT Sport 2 HD 138 Universal -

History of Antibiotics
Sumerianz Journal of Medical and Healthcare, 2018, Vol. 1, No. 2, pp. 51-54 ISSN(e): 2663-421X, ISSN(p): 2706-8404 Website: https://www.sumerianz.com © Sumerianz Publication CC BY: Creative Commons Attribution License 4.0 Original Article Open Access History of Antibiotics Kourkouta L.* Professor of Nursing Department, Alexander Technological Educational Institute of Thessaloniki, Greece Tsaloglidou A. Assistant Professor of Nursing Department, Alexander Technological Educational Institute of Thessaloniki, Greece Koukourikos K. Clinical Professor of Nursing Department, Alexander Technological Educational Institute of Thessaloniki, Greece Iliadis C. RN, Private Diagnostic Health Center of Thessaloniki, Greece Plati P. Department of History and Archaeology, Greece Dimitriadou A. Professor of Nursing Department, Alexander Technological Educational Institute of Thessaloniki, Greece Abstract Introduction: Antibiotics are medicines used to treat or prevent bacterial infections. They can either kill or inhibit the growth of bacteria. Aim: The aim of this historical review is to provide information on the discovery and use of antibiotic formulations over time. Review Methods: The study material consisted of scientific publications related to the subject, such as those recorded in the literature of this study, and relevant writings. Results: The first known use of antibiotics was by the ancient Chinese over 2,500 years ago. Chinese have discovered the therapeutic properties of moldy soybeans and used this substance to cure furuncles (pimples), carbuncles and similar infections. Sir Alexander Fleming was a Scottish biologist and pharmacologist and he was involved in research of Bacteriology, Immunology and Chemotherapy. He is well known for the discovery of the first antibiotic, penicillin, in 1928, for which he received the Nobel Prize in Physiology and Medicine in 1945, along with Florey and Chain.