A Determination of Phylogeny and Hybridization History Within Clematis L. (Ranunculaceae) Using Actin and Nitrate Reductase Intr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Approaches and Limitations of Species Level Diagnostics in Flowering Plants
Genetic Food Diagnostics Approaches and Limitations of Species Level Diagnostics in Flowering Plants Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) Fakultät für Chemie und Biowissenschaften Karlsruher Institut für Technologie (KIT) - Universitätsbereich genehmigte DISSERTATION von Dipl. Biologe Thomas Horn aus 77709 Wolfach Dekan: Prof. Dr. Peter Roesky Referent: Prof. Dr. Peter Nick Korreferent: Prof. Dr. Horst Taraschewski Tag der mündlichen Prüfung: 17.04.2014 Parts of this work are derived from the following publications: Horn T, Völker J, Rühle M, Häser A, Jürges G, Nick P; 2013; Genetic authentication by RFLP versus ARMS? The case of Moldavian Dragonhead (Dracocephalum moldavica L.). European Food Research and Technology, doi 10.1007/s00217-013-2089-4 Horn T, Barth A, Rühle M, Häser A, Jürges G, Nick P; 2012; Molecular Diagnostics of Lemon Myrtle (Backhousia citriodora versus Leptospermum citratum). European Food Research and Technology, doi 10.1007/s00217-012-1688-9 Also included are works from the following teaching projects: RAPD Analysis and SCAR design in the TCM complex Clematis Armandii Caulis (chuān mù tōng), F2 Plant Evolution, 2011 Effects of highly fragmented DNA on PCR, F3, Lidija Krebs, 2012 1 I. Acknowledgement “Nothing is permanent except change” Heraclitus of Ephesus Entering adolescence – approximately 24 years ago – many aspects of life pretty much escaped my understanding. After a period of turmoil and subsequent experience of a life as laborer lacking an education, I realized that I did not want to settle for this kind of life. I wanted to change. With this work I would like to thank all people that ever bothered trying to explain the world to me, that allowed me to find my way and nurtured my desire to change. -

Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Spring 5-2016 Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi Hanna M. Miller University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Biodiversity Commons, and the Botany Commons Recommended Citation Miller, Hanna M., "Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi" (2016). Honors Theses. 389. https://aquila.usm.edu/honors_theses/389 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi by Hanna Miller A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirement for the Degree of Bachelor of Science in the Department of Biological Sciences May 2016 ii Approved by _________________________________ Mac H. Alford, Ph.D., Thesis Adviser Professor of Biological Sciences _________________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences _________________________________ Ellen Weinauer, Ph.D., Dean Honors College iii Abstract The North American Coastal Plain contains some of the highest plant diversity in the temperate world. However, most of the region has remained unstudied, resulting in a lack of knowledge about the unique plant communities present there. -
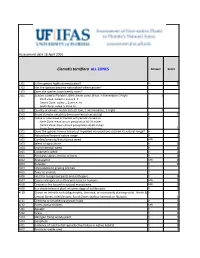
WRA.Datasheet.Template (Version 1) (Version 1).Xlsx
Assessment date 18 April 2016 Clematis terniflora ALL ZONES Answer Score 1.01 Is the species highly domesticated? n 0 1.02 Has the species become naturalised where grown? 1.03 Does the species have weedy races? 2.01 Species suited to Florida's USDA climate zones (0-low; 1-intermediate; 2-high) 2 North Zone: suited to Zones 8, 9 Central Zone: suited to Zones 9, 10 South Zone: suited to Zone 10 2.02 Quality of climate match data (0-low; 1-intermediate; 2-high) 2 2.03 Broad climate suitability (environmental versatility) y 1 2.04 Native or naturalized in habitats with periodic inundation y North Zone: mean annual precipitation 50-70 inches Central Zone: mean annual precipitation 40-60 inches South Zone: mean annual precipitation 40-60 inches 1 2.05 Does the species have a history of repeated introductions outside its natural range? y 3.01 Naturalized beyond native range y 2 3.02 Garden/amenity/disturbance weed unk 3.03 Weed of agriculture n 0 3.04 Environmental weed y 4 3.05 Congeneric weed y 2 4.01 Produces spines, thorns or burrs n 0 4.02 Allelopathic unk 0 4.03 Parasitic n 0 4.04 Unpalatable to grazing animals ? 4.05 Toxic to animals ? 4.06 Host for recognised pests and pathogens n 0 4.07 Causes allergies or is otherwise toxic to humans unk 0 4.08 Creates a fire hazard in natural ecosystems unk 0 4.09 Is a shade tolerant plant at some stage of its life cycle n 0 4.10 Grows on infertile soils (oligotrophic, limerock, or excessively draining soils). -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Download Curriculum Vitae
Jason Ager Koontz Biology Department, Augustana College Phone: 309-794-3442 639-38th Street FAX: 309-794-8004 Rock Island, IL 61201 E-mail: [email protected] Education 1993 B.S. (Botany) Iowa State University, Ames, IA (with Distinction, Honors Program, and Phi Beta Kappa) 1995 M.S. (Botany) Miami University, Oxford, OH 2000 Ph.D. (Botany) Washington State University, Pullman, WA Current Position 7/14-present: Chair of Biology 8/11-7/14: Co-Chair of Biology 8/10: Tenured and promoted to Associate Professor 9/04-8/10: Assistant Professor of Biology Becoming Biologists (BI150), General Botany (BI220), Cell Biology (BI210), Nutrition (BI263; 2004-2006), Natural History of Ireland (BI328; 2010, 2013), Conservation Biology (BI410), Conservation Biology Senior Inquiry (BI464) Non-Academic Positions 5/12-present: Research Associate, Rancho Santa Ana Botanic Garden, Claremont, CA. 1/06-present: Research Associate, Department of Botany, The Field Museum of Natural History, Chicago, IL. 10/04-present: Adjunct Assistant Professional Scientist, Illinois Natural History Survey, Prairie Research Institute, University of Illinois Urbana-Champaign, IL. 5/00-9/04: Assistant Research Scientist III, Plant Systematist, Centers for Biodiversity and Wildlife and Plant Ecology, Illinois Natural History Survey, Champaign, IL. Academic Positions 10/01-12/07: Affiliate Assistant Professor, Department of Plant Biology, University of Illinois at Urbana-Champaign, IL. 8/95-5/00: Graduate Teaching Assistant, Department of Botany, Washington State University, -

Genetic Variation in the Illinois-Threatened Hill Prairie Larkspur
GENETIC VARIATION IN THE ILLINOIS-THREATENED HiLL PRAIRIE LARKSPUR Brooke BRYant, Department of Biology, Augustana College, 639-38th Street, Rock Island, IL 61201 EliZabeth EYler, Illinois College of Optometry, 3241 S. Michigan Avenue, Chicago, IL 60616 Stefan Johnsrud, Department of Plant Biology, University of Illinois at Urbana-Champaign, 265 Morrill Hall, 505 S. Goodwin Avenue, Urbana, IL 61801 Jason KoontZ, Department of Biology, Augustana College, 639-38th Street, Rock Island, IL 61201, [email protected] Abstract: Delphinium carolinianum Walter is a threatened A hill prairie is a dry grassland patch located on the west wildflower in the state of Illinois, where it is limited to iso- or southwest slope of an elevation (Evers 1955). Hill prai- lated hill prairies along the Mississippi River. Isolated pop- ries are one of the least disturbed prairies left in the United ulations often experience little to no gene flow with other States because the steep slopes where they are located are populations, and this may cause inbreeding depression that not suitable for conversion to farmland. Agriculture and ultimately leads to extinction. Through use of amplified development have replaced the surrounding natural areas, fragment length polymorphism (AFLP) molecular markers, causing the habitat of the hill prairie larkspur to become we assessed the patterns of genetic variation within and be- highly fragmented into small “hill prairie islands.” The tween populations of D. carolinianum to better understand lack of conservation within and around the hill prairies the stability of extant populations of this rare wildflower. Jo Daviess Stephenson Our data show that high levels of genetic variation are pres- Winnebago Boone McHenr y Lake ent within the populations studied, yet it is unclear whether Carroll Ogle DeKalb Kane Cook this variation is due to current gene flow or preservation of DuPage Whiteside Lee historic variation. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2012
Natural Heritage Program List of Rare Plant Species of North Carolina 2012 Edited by Laura E. Gadd, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program Office of Conservation, Planning, and Community Affairs N.C. Department of Environment and Natural Resources 1601 MSC, Raleigh, NC 27699-1601 Natural Heritage Program List of Rare Plant Species of North Carolina 2012 Edited by Laura E. Gadd, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program Office of Conservation, Planning, and Community Affairs N.C. Department of Environment and Natural Resources 1601 MSC, Raleigh, NC 27699-1601 www.ncnhp.org NATURAL HERITAGE PROGRAM LIST OF THE RARE PLANTS OF NORTH CAROLINA 2012 Edition Edited by Laura E. Gadd, Botanist and John Finnegan, Information Systems Manager North Carolina Natural Heritage Program, Office of Conservation, Planning, and Community Affairs Department of Environment and Natural Resources, 1601 MSC, Raleigh, NC 27699-1601 www.ncnhp.org Table of Contents LIST FORMAT ......................................................................................................................................................................... 3 NORTH CAROLINA RARE PLANT LIST ......................................................................................................................... 10 NORTH CAROLINA PLANT WATCH LIST ..................................................................................................................... 71 Watch Category -

Annotated Checklist of the Butterflies of Bentsen-Rio Grande Valley State
AN ANNOTATED CHECKLIST OF THE BUTTERFLIES (LEPIDOPTERA: RHOPALOCERA) OF BENTSEN-RIO GRANDE STATE VALLEY PARK AND VICINITY JUNE, 1974 Published by TEXAS PARKS & WILDLIFE DEPARTMENT BENTSEN-RIO GRANDE VALLEY STATE PARK P.O. 30X 988; MISSION, TEXAS 78572 INTRODUCTION The species listed here in are primarily a result of the collecting by the authors during the period 1972-1973. Certain important records of the previous several years are also included. Additionally, the checklist incorporates records of a number of other lepidopterists. The primary focus of the checklist, then, is upon recent collecting, rather than being an attempt to list all known records from the Mid-Valley area. All lepidopterists collecting in the park and vicinity are urged to send copies of their records to the authors and/or the park authorities. A number of species on the list have been taken in Hidalgo Co. but not yet within the actual confines of the park; the annotations will indicate which species these are. Some of these have been taken at Santa Ana National Wildlife Refuge, approximately thirty miles down river, in habitats similar to those within the park. Others have been taken within several miles of the park, in nearby towns and along roadsides. These species can be reasonably expected to occur in the park, and their inclusion upon this list should alert the collector to their possible presence. The annotations have been kept necessarily brief. They are intended to aid the visiting lepidopterist in evaluating the significance of his catches. Local larval food plants are given where known. Much, however, is still to be learned regarding the life histories of even some of the commoner species. -

David A. Rasmussen, 2 Elena M. Kramer, 3 and Elizabeth A. Zimmer 4
American Journal of Botany 96(1): 96–109. 2009. O NE SIZE FITS ALL? M OLECULAR EVIDENCE FOR A COMMONLY INHERITED PETAL IDENTITY PROGRAM IN RANUNCULALES 1 David A. Rasmussen, 2 Elena M. Kramer, 3 and Elizabeth A. Zimmer 4 Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts 02138 USA Petaloid organs are a major component of the fl oral diversity observed across nearly all major clades of angiosperms. The vari- able morphology and development of these organs has led to the hypothesis that they are not homologous but, rather, have evolved multiple times. A particularly notable example of petal diversity, and potential homoplasy, is found within the order Ranunculales, exemplifi ed by families such as Ranunculaceae, Berberidaceae, and Papaveraceae. To investigate the molecular basis of petal identity in Ranunculales, we used a combination of molecular phylogenetics and gene expression analysis to characterize APETALA3 (AP3 ) and PISTILLATA (PI ) homologs from a total of 13 representative genera of the order. One of the most striking results of this study is that expression of orthologs of a single AP3 lineage is consistently petal-specifi c across both Ranunculaceae and Berberidaceae. We conclude from this fi nding that these supposedly homoplastic petals in fact share a developmental genetic program that appears to have been present in the common ancestor of the two families. We discuss the implications of this type of molecular data for long-held typological defi nitions of petals and, more broadly, the evolution of petaloid organs across the angiosperms. Key words: APETALA3 ; MADS box genes; petal evolution; PISTILLATA ; Ranunculales. -

Species List For: Valley View Glades NA 418 Species
Species List for: Valley View Glades NA 418 Species Jefferson County Date Participants Location NA List NA Nomination and subsequent visits Jefferson County Glade Complex NA List from Gass, Wallace, Priddy, Chmielniak, T. Smith, Ladd & Glore, Bogler, MPF Hikes 9/24/80, 10/2/80, 7/10/85, 8/8/86, 6/2/87, 1986, and 5/92 WGNSS Lists Webster Groves Nature Study Society Fieldtrip Jefferson County Glade Complex Participants WGNSS Vascular Plant List maintained by Steve Turner Species Name (Synonym) Common Name Family COFC COFW Acalypha virginica Virginia copperleaf Euphorbiaceae 2 3 Acer rubrum var. undetermined red maple Sapindaceae 5 0 Acer saccharinum silver maple Sapindaceae 2 -3 Acer saccharum var. undetermined sugar maple Sapindaceae 5 3 Achillea millefolium yarrow Asteraceae/Anthemideae 1 3 Aesculus glabra var. undetermined Ohio buckeye Sapindaceae 5 -1 Agalinis skinneriana (Gerardia) midwestern gerardia Orobanchaceae 7 5 Agalinis tenuifolia (Gerardia, A. tenuifolia var. common gerardia Orobanchaceae 4 -3 macrophylla) Ageratina altissima var. altissima (Eupatorium rugosum) white snakeroot Asteraceae/Eupatorieae 2 3 Agrimonia pubescens downy agrimony Rosaceae 4 5 Agrimonia rostellata woodland agrimony Rosaceae 4 3 Allium canadense var. mobilense wild garlic Liliaceae 7 5 Allium canadense var. undetermined wild garlic Liliaceae 2 3 Allium cernuum wild onion Liliaceae 8 5 Allium stellatum wild onion Liliaceae 6 5 * Allium vineale field garlic Liliaceae 0 3 Ambrosia artemisiifolia common ragweed Asteraceae/Heliantheae 0 3 Ambrosia bidentata lanceleaf ragweed Asteraceae/Heliantheae 0 4 Ambrosia trifida giant ragweed Asteraceae/Heliantheae 0 -1 Amelanchier arborea var. arborea downy serviceberry Rosaceae 6 3 Amorpha canescens lead plant Fabaceae/Faboideae 8 5 Amphicarpaea bracteata hog peanut Fabaceae/Faboideae 4 0 Andropogon gerardii var. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY BIBLIOGRAPHY Ackerfield, J., and J. Wen. 2002. A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. Adansonia 24: 197-212. Adams, P. 1961. Observations on the Sagittaria subulata complex. Rhodora 63: 247-265. Adams, R.M. II, and W.J. Dress. 1982. Nodding Lilium species of eastern North America (Liliaceae). Baileya 21: 165-188. Adams, R.P. 1986. Geographic variation in Juniperus silicicola and J. virginiana of the Southeastern United States: multivariant analyses of morphology and terpenoids. Taxon 35: 31-75. ------. 1995. Revisionary study of Caribbean species of Juniperus (Cupressaceae). Phytologia 78: 134-150. ------, and T. Demeke. 1993. Systematic relationships in Juniperus based on random amplified polymorphic DNAs (RAPDs). Taxon 42: 553-571. Adams, W.P. 1957. A revision of the genus Ascyrum (Hypericaceae). Rhodora 59: 73-95. ------. 1962. Studies in the Guttiferae. I. A synopsis of Hypericum section Myriandra. Contr. Gray Herbarium Harv. 182: 1-51. ------, and N.K.B. Robson. 1961. A re-evaluation of the generic status of Ascyrum and Crookea (Guttiferae). Rhodora 63: 10-16. Adams, W.P. 1973. Clusiaceae of the southeastern United States. J. Elisha Mitchell Sci. Soc. 89: 62-71. Adler, L. 1999. Polygonum perfoliatum (mile-a-minute weed). Chinquapin 7: 4. Aedo, C., J.J. Aldasoro, and C. Navarro. 1998. Taxonomic revision of Geranium sections Batrachioidea and Divaricata (Geraniaceae). Ann. Missouri Bot. Gard. 85: 594-630. Affolter, J.M. 1985. A monograph of the genus Lilaeopsis (Umbelliferae). Systematic Bot. Monographs 6. Ahles, H.E., and A.E.