Cusk,Brosme Brosme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Concept for Assuring the Quality of Seafoods to Consumers
MFR PAPER 1276 Table I.-The approximate shelf life of cod fillets (Ronalvalll et aI., 1973). Temperalure Approximate OF 0C shelf file (days) 32 0 14 A Concept for Assuring the Quality of 34 1.11 11 37 2.78 8 39 3.89 7 Seafoods to Consumers 41 5.00 6 44 6.67 5 49 9.44 4 56 13.30 3 L. J. RONSIVALLI, C. GORGA, J. D. KAYLOR, and J. H. CARVER at 31°_33°F (-0.56°-0.56°C) is about 2 weeks from the time they are har vested. (The shelf life of a product, in ABSTRACT - For more than a decade numerous surveys of the quality of this case, ends when it becomes unac seafoods at retail counters have resulted in consistent adverse reports, and it has ceptable as a food; that is, the total time been concluded that the unreliability of the quality of seafoods is the major reason it takes for the product to change from why their per capita consumption is so much lower than it is for beef, pork, or Grade A to Grade B to Grade C and then poultry. A concept for insuring the Grade A quality ofseafoods was developed and to the point just before it becomes unac tested in an attempt to demonstrate that the consumption offresh fish will increase ceptable.)' It has been established that when their quality and image are improved. This paper describes the concept, its the shelf life of fresh seafoods is af implementation under federal coordination, and its successful implementation by fected mainly by the temperature at industry which has grown from a pilot operation involving five retail stores and which they are held (Table 1). -

Histological Aspects of the Early Development of the Digestive System of Burbot Lota Lota L
ISSN 0015-5497, e-ISSN 1734-9168 Folia Biologica (Kraków), vol. 64 (2016), No 1 Ó Institute of Systematics and Evolution of Animals, PAS, Kraków, 2015 doi:10.3409/fb64_1.11 Histological Aspects of the Early Development of the Digestive System of Burbot Lota lota L. (Lotidae, Gadiformes)* Gra¿yna FURGA£A-SELEZNIOW, Ma³gorzata JANKUN, Roman KUJAWA, Joanna NOWOSAD, Maria BI£AS, Dariusz KUCHARCZYK, and Andrzej SKRZYPCZAK Accepted December 04, 2015 Published January 29, 2016 FURGA£A-SELEZNIOW G., JANKUN M., KUJAWA R., NOWOSAD J., BI£AS M., KUCHARCZYK D., SKRZYPCZAK A. 2016. Histological aspects of the early development of the digestive system of burbot Lota lota L. (Lotidae, Gadiformes). Folia Biologica (Kraków) 64: 11-21. The ontogeny of the digestive tract was studied histologically in burbot, Lota lota L., from hatching to 42 days post-hatch (dph). At hatching, the digestive tract consisted of a straight tube with discernible digestive accessory glands (the liver and the pancreas) dorsally attached to the yolk sac. Most of the yolk sac reserves were consumed during the first 12 days and were completely depleted by 17 dph. The first PAS-positive goblet cells appeared at 6 dph, dispersed within the epithelium of the oesophagus and increasing substantially in number and distribution as development progressed. At 12 dph, the first vacuoles (neutral lipids) appeared in the intestine, indicating the functional absorption of nutrients from food. Differentiation of gastric glands was first noticed at 17 dph and was extensive by 27 dph. L. lota larvae have a morphologically complete digestive tract by 32 dph. -

Foreign Fishery Trade
August 1946 COMMERCIAL FISHERIES REVIEW 31 FOREIGN FISHERY TRADE Imports and Exports GROUNDFISH IMPORTS: From January 1 through J~e 29,1946, there were 24,676,000 pounds of fresh and frozen groundfish imported into the United States under the special tariff classification "Fish, fresh or frozen fillets, steaks, etc., of cod, haddock, hake, cusk, pollock, and rosefish." Approximately 19,615,000 pounds were received during the corresponding period in 1945, according to a report re ceived from the Bureau of Customs of the Treasury Department. The reduced tariff quota for the year is 20,380,724 pounds. June 2-29, Apr. 'LJ- June Jan. 1- Jan. 1- Commodity 1946 June 1.1946 19A5 June _29_ l~6 June J.o 1'3A5. Fish, fresh or frozen fillets, steaks, etc•• I of cod, haddock, hake, 4,330,976 3,983,146 3,176,093 24,675,749 19.615,133 cusk, pollock, and rosefish Canada COLD~STORAGE: Canadian freezings of fresh fishery products in May totaled 14,422,000 pounds. HeaViest freezings were of whole cod, cod fil~ets, and halibut, according to a preliminary report of the Department of Trade and Commerce of the DOminion Bureau of Statistics. Holdings totaled 22,309,000 pounds on June 1, compared with 15,537,000 pounds on May 1 and 17,489,000 pounds on June 1, 1945. Ecuador FISHING REGULATION: A report trom the American Embassy at ~uito, Ecuador, received by the State Department on July 1, 1946, states that the President of Ecuador, on December 27, 1945, signed Exeoutive Decree No. -

CHAPTER 3 FISH and CRUSTACEANS, MOLLUSCS and OTHER AQUATIC INVERTEBRATES I 3-L Note
)&f1y3X CHAPTER 3 FISH AND CRUSTACEANS, MOLLUSCS AND OTHER AQUATIC INVERTEBRATES I 3-l Note 1. This chapter does not cover: (a) Marine mammals (heading 0106) or meat thereof (heading 0208 or 0210); (b) Fish (including livers and roes thereof) or crustaceans, molluscs or other aquatic invertebrates, dead and unfit or unsuitable for human consumption by reason of either their species or their condition (chapter 5); flours, meals or pellets of fish or of crustaceans, molluscs or other aquatic invertebrates, unfit for human consumption (heading 2301); or (c) Caviar or caviar substitutes prepared from fish eggs (heading 1604). 2. In this chapter the term "pellets" means products which have been agglomerated either directly by compression or by the addition of a small quantity of binder. Additional U.S. Note 1. Certain fish, crustaceans, molluscs and other aquatic invertebrates are provided for in chapter 98. )&f2y3X I 3-2 0301 Live fish: 0301.10.00 00 Ornamental fish............................... X....... Free Free Other live fish: 0301.91.00 00 Trout (Salmo trutta, Salmo gairdneri, Salmo clarki, Salmo aguabonita, Salmo gilae)................................... X....... Free Free 0301.92.00 00 Eels (Anguilla spp.)..................... kg...... Free Free 0301.93.00 00 Carp..................................... X....... Free Free 0301.99.00 00 Other.................................... X....... Free Free 0302 Fish, fresh or chilled, excluding fish fillets and other fish meat of heading 0304: Salmonidae, excluding livers and roes: 0302.11.00 Trout (Salmo trutta, Salmo gairdneri, Salmo clarki, Salmo aguabonita, Salmo gilae)................................... ........ Free 2.2¢/kg 10 Rainbow trout (Salmo gairnderi), farmed.............................. kg 90 Other............................... kg 0302.12.00 Pacific salmon (Oncorhynchus spp.), Atlantic salmon (Salmo salar) and Danube salmon (Hucho hucho)............. -

Marine Fishes from Galicia (NW Spain): an Updated Checklist
1 2 Marine fishes from Galicia (NW Spain): an updated checklist 3 4 5 RAFAEL BAÑON1, DAVID VILLEGAS-RÍOS2, ALBERTO SERRANO3, 6 GONZALO MUCIENTES2,4 & JUAN CARLOS ARRONTE3 7 8 9 10 1 Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca 11 e Asuntos Marítimos, Rúa do Valiño 63-65, 15703 Santiago de Compostela, Spain. E- 12 mail: [email protected] 13 2 CSIC. Instituto de Investigaciones Marinas. Eduardo Cabello 6, 36208 Vigo 14 (Pontevedra), Spain. E-mail: [email protected] (D. V-R); [email protected] 15 (G.M.). 16 3 Instituto Español de Oceanografía, C.O. de Santander, Santander, Spain. E-mail: 17 [email protected] (A.S); [email protected] (J.-C. A). 18 4Centro Tecnológico del Mar, CETMAR. Eduardo Cabello s.n., 36208. Vigo 19 (Pontevedra), Spain. 20 21 Abstract 22 23 An annotated checklist of the marine fishes from Galician waters is presented. The list 24 is based on historical literature records and new revisions. The ichthyofauna list is 25 composed by 397 species very diversified in 2 superclass, 3 class, 35 orders, 139 1 1 families and 288 genus. The order Perciformes is the most diverse one with 37 families, 2 91 genus and 135 species. Gobiidae (19 species) and Sparidae (19 species) are the 3 richest families. Biogeographically, the Lusitanian group includes 203 species (51.1%), 4 followed by 149 species of the Atlantic (37.5%), then 28 of the Boreal (7.1%), and 17 5 of the African (4.3%) groups. We have recognized 41 new records, and 3 other records 6 have been identified as doubtful. -
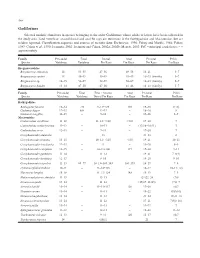
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Brosme Brosme
Cusk − Brosme brosme Overall Vulnerability Rank = High Biological Sensitivity = High Climate Exposure = High Data Quality = 67% of scores ≥ 2 Expert Data Expert Scores Plots Scores Quality (Portion by Category) Brosme brosme Low Moderate Stock Status 3.0 0.4 High Other Stressors 1.5 0.8 Very High Population Growth Rate 3.1 1.6 Spawning Cycle 3.0 2.4 Complexity in Reproduction 1.3 1.5 Early Life History Requirements 2.6 0.2 Sensitivity to Ocean Acidification 1.2 1.6 Prey Specialization 1.1 2.4 Habitat Specialization 2.7 2.4 Sensitivity attributes Sensitivity to Temperature 2.0 2.4 Adult Mobility 2.7 2.4 Dispersal & Early Life History 1.8 2.2 Sensitivity Score High Sea Surface Temperature 3.9 3.0 Variability in Sea Surface Temperature 1.0 3.0 Salinity 1.1 3.0 Variability Salinity 1.2 3.0 Air Temperature 1.0 3.0 Variability Air Temperature 1.0 3.0 Precipitation 1.0 3.0 Variability in Precipitation 1.0 3.0 Ocean Acidification 4.0 2.0 Exposure variables Variability in Ocean Acidification 1.0 2.2 Currents 2.1 1.0 Sea Level Rise 1.1 1.5 Exposure Score High Overall Vulnerability Rank High Cusk (Brosme brosme) Overall Climate Vulnerability Rank: High (69% certainty from bootstrap analysis). Climate Exposure: High. Two exposure factors contributed to this score: Ocean Surface Temperature (3.9) and Ocean Acidification (4.0). All life stages of Cusk use marine habitats. Biological Sensitivity: High. Three sensitivity attributes scored above 3.0: Stock Status (3.0), Population Growth Rate (3.1), and Spawning Cycle (3.0). -

Cusk (Brosme Brosme)
Cusk (Brosme brosme) Family Gadidae, Cods Common name: cusk Description: Cusk are dark slate to red brown above with yellowish sides and a dirty white underbody. They have an elongated, taper shaped body with a blunt snout and a single barbel on their chin. Their dorsal and anal fins are exceptionally long and they have a rounded tail fin, all of which are bordered with a black stripe that is edged in white. Cusk can grow to a size of about 3 1/2 feet in length and to 30 pounds in weight. Their average size in the waters of the Gulf of Maine, however, is closer to 1 1/2 to 2 1/2 feet in length and 5 to 10 pounds in weight. Where found: offshore Similar Gulf of Maine species: wolffish, eel pout Remarks: Cusk are exclusively bottom dwellers that inhabit moderately deep waters of 60 to 90 feet. They prefer a hard rough substrate made up of rocks or boulders. These fish are solitary in nature and are not particularly abundant. Cusk are excellent table fare, particularly in chowders and stews. Occasionally, anglers will hook onto a cusk while fishing for cod or haddock. Although these fish are considered to be weak and sluggish swimmers, they have a powerful body. Both clams and herring work well as bait when fishing for cusk. Records: MSSAR IGFA AllTackle World Record Fish Illustrations by: Roz Davis Designs, Damariscotta, ME (207) 5632286 With permission, the use of these pictures must state the following: Drawings provided courtesy of the Maine Department of Marine Resources Recreational Fisheries program and the Maine Outdoor Heritage Fund.. -

The Complete Mitochondrial Genome of the Whiting, Merlangius
CORE Metadata, citation and similar papers at core.ac.uk Provided by Digital.CSIC The complete mitochondrial genome of the whiting, Merlangius merlangus and the haddock, Melanogrammus aeglefinus: A detailed genomic comparison among closely related species of the Gadidae family Severine Roques a , Clive J. Fox b , Maria I. Villasana a , Ciro Rico a,⁎ a Estación Biológica de Doñana, CSIC, Pabellón del Perú, 41013 Sevilla, Spain b The Centre for Environment, Fisheries and Aquaculture Science, Pakefield Road, Lowestoft, Suffolk, NR33 OHT, England, United Kingdom Abstract We determined the first complete mitochondrial DNA (mtDNA) sequences for the whiting (Merlangius merlangus, family Gadidae, order Gadiformes) and the haddock (Melanogrammus aeglefinus, family Gadidae, order Gadiformes). The entire mitogenomes were amplified and sequenced by primer walking using newly designed specific internal primers. Lengths were 16,569 and 16,585 bases for whiting and haddock respectively, lengths which lie within the range of previously reported gadiform sequences from Atlantic cod (Gadus morhua, 16,696 bases) and walleye pollock (Theragra chalcogramma, 16,570 bases). Gene arrangement in both species conformed to the order seen in most vertebrate mitochondrial genomes. We identified a long intergenic spacer located between the tRNAThr and tRNAPro genes (of 100 and 70 bp long for whiting and haddock, respectively), as previously described for other species of the order Gadiformes. Using nucleotide and amino acid divergence data of four complete gadoid mitogenomes (M. merlangius, M. aeglefinus, G. morhua and T. chalcogramma), we examined in detail the relative mtDNA mutation patterns across genes and among Gadidae species and tested for the performance of each protein-coding, transfer RNA and ribosomal RNA gene in depicting the expected phylogeny among the four species, as compared with the whole genome dataset. -

Length-Weight Relationships of Marine Fish Collected from Around the British Isles
Science Series Technical Report no. 150 Length-weight relationships of marine fish collected from around the British Isles J. F. Silva, J. R. Ellis and R. A. Ayers Science Series Technical Report no. 150 Length-weight relationships of marine fish collected from around the British Isles J. F. Silva, J. R. Ellis and R. A. Ayers This report should be cited as: Silva J. F., Ellis J. R. and Ayers R. A. 2013. Length-weight relationships of marine fish collected from around the British Isles. Sci. Ser. Tech. Rep., Cefas Lowestoft, 150: 109 pp. Additional copies can be obtained from Cefas by e-mailing a request to [email protected] or downloading from the Cefas website www.cefas.defra.gov.uk. © Crown copyright, 2013 This publication (excluding the logos) may be re-used free of charge in any format or medium for research for non-commercial purposes, private study or for internal circulation within an organisation. This is subject to it being re-used accurately and not used in a misleading context. The material must be acknowledged as Crown copyright and the title of the publication specified. This publication is also available at www.cefas.defra.gov.uk For any other use of this material please apply for a Click-Use Licence for core material at www.hmso.gov.uk/copyright/licences/ core/core_licence.htm, or by writing to: HMSO’s Licensing Division St Clements House 2-16 Colegate Norwich NR3 1BQ Fax: 01603 723000 E-mail: [email protected] 3 Contents Contents 1. Introduction 5 2. -

Establishment and Partial Characterization of a Cell Line from Burbot Lota Lota Maculosa: Susceptibility to IHNV, IPNV and VHSV Mark P
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- ubP lished Research US Geological Survey 5-18-2010 Establishment and partial characterization of a cell line from burbot Lota lota maculosa: susceptibility to IHNV, IPNV and VHSV Mark P. Polinski Department of Fish and Wildlife Resources, College of Natural Resources, University of Idaho John D. Drennan Intervet/Schering-Plough Animal Health William N. Batts US Geological Survey, Western Fisheries Research Center Susan C. Ireland Kootenai Tribe of Idaho Kenneth D. Cain Department of Fish and Wildlife Resources, College of Natural Resources, University of Idaho,, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/usgsstaffpub Part of the Geology Commons, Oceanography and Atmospheric Sciences and Meteorology Commons, Other Earth Sciences Commons, and the Other Environmental Sciences Commons Polinski, Mark P.; Drennan, John D.; Batts, iW lliam N.; Ireland, Susan C.; and Cain, Kenneth D., "Establishment and partial characterization of a cell line from burbot Lota lota maculosa: susceptibility to IHNV, IPNV and VHSV" (2010). USGS Staff -- Published Research. 1062. http://digitalcommons.unl.edu/usgsstaffpub/1062 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- ubP lished Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Vol. 90: 15–23, 2010 DISEASES OF AQUATIC ORGANISMS Published May 18 doi: 10.3354/dao02215 Dis Aquat Org Establishment and partial characterization of a cell line from burbot Lota lota maculosa: susceptibility to IHNV, IPNV and VHSV Mark P. -

Comparison of Size Selectivity Between Marine Mammals and Commercial Fisheries with Recommendations for Restructuring Management Policies
NOAA Technical Memorandum NMFS-AFSC-159 Comparison of Size Selectivity Between Marine Mammals and Commercial Fisheries with Recommendations for Restructuring Management Policies by M. A. Etnier and C. W. Fowler U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center October 2005 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Etnier, M. A., and C. W. Fowler. 2005. Comparison of size selectivity between marine mammals and commercial fisheries with recommendations for restructuring management policies. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-159, 274 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-159 Comparison of Size Selectivity Between Marine Mammals and Commercial Fisheries with Recommendations for Restructuring Management Policies by M. A. Etnier and C. W. Fowler Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle, WA 98115 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE Carlos M.